The Evolution of Traditional Chinese Medicine in Pharmaceutical Packaging

How high-speed tablet machine revolutionize production?
4 Types of Veterinary Medicine Formulations: Which One Suits Your Needs?
Introduction
Veterinary medicine is a field that continually evolves to meet the diverse needs of animal health care. In today’s competitive market, formulation technology plays a vital role in ensuring safety, efficacy, and compliance. With modern equipment such as the Solid Preparation Granulation Line and state-of-the-art Pharma Packaging systems, pharmaceutical plants and laboratories can now produce high-quality pet medicine and other veterinary products efficiently. In this post, we examine four main types of veterinary formulations and discuss how advanced packaging technologies support each category.
Source: pixabay
1. Solid Dosage Forms
Solid formulations are the backbone of veterinary medicine. They include tablets, capsules, powders, and granules that are us ed widely across the industry.
Key Features of Solid Dosage Forms:
Tablets:

Tablets are compressed solid forms that may include various subtypes such as:
- Effervescent Tablets: Designed to dissolve rapidly in water, releasing active ingredients quickly.
- Sustained-Release and Controlled-Release Tablets: Engineered for gradual drug release, ensuring prolonged therapeutic effects.
- Enteric-Coated Tablets: These tablets have a protective layer that prevents drug degradation in the stomach and allows release in the intestines.
Capsules:

Capsules can be either hard or soft, filled with powders or granulated ingredients. They come in various forms including:
- Hard Capsules: Generally used for powders or granules.
- Soft Capsules: Typically contain liquid formulations or suspensions.
- Modified Release Capsules: Designed to release their contents slowly, matching specific treatment needs.
Powders and Granules:

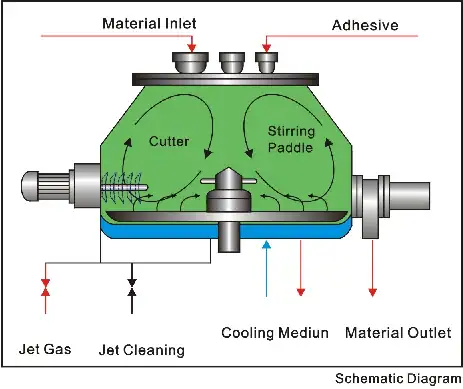
High-Efficiency Three-Dimensional Pharmaceutical Mixer
Powders are finely milled ingredients, while granules have a coarser texture. Granules are particularly beneficial in veterinary applications because:
- They are easy to measure and mix.
- They are often processed on a Solid Preparation Granulation Line, which ensures uniform particle size and consistent dosage.
Solid formulations offer excellent stability and can be easily stored, making them an attractive option for veterinary medicine manufacturers, whether in a laboratory setting or a full-scale pharmaceutical plant.
2. Liquid Dosage Forms
Liquid formulations are essential when rapid absorption or ease of administration is critical. They are commonly used in injectable medications, oral solutions, and topical liquids.
Common Liquid Dosage Forms:
Injectables:
These include solutions, emulsions, and suspensions prepared under sterile conditions. They are crucial for:- Immediate therapeutic effects.
- Direct administration via intramuscular or intravenous routes.
Oral Liquid Formulations:
These are typically solutions, suspensions, or emulsions intended for direct oral consumption. They provide benefits such as:- Ease of dosing, especially for young or uncooperative animals.
- Rapid absorption and quick onset of action.
Topical Liquids:
Used for external applications, these formulations can be applied directly on the skin or mucous membranes.- They help treat localized infections or inflammations.
- They are often packaged in bottles or specialized containers through advanced Pharma Packaging systems to ensure precision and sterility.
Liquid dosage forms are particularly advantageous when the veterinary treatment requires swift therapeutic action. They can be produced efficiently in both small laboratory batches and large-scale pharmaceutical plants.
3. Semi-Solid Dosage Forms
Semi-solid formulations include ointments, creams, gels, and pastes. These are primarily used for topical applications and provide a sustained release of the active ingredient at the site of application.
Benefits of Semi-Solid Formulations:
Ointments and Creams:
These are mixed with appropriate oil or water bases and are used for external treatment. They are designed to:- Provide a protective barrier.
- Allow for gradual absorption of the drug.
- Be easily applied to affected areas, making them ideal for skin conditions in animals.
Gels and Pastes:
These semi-solid forms are particularly effective for:- Localized treatment.
- Extended drug contact time.
- Reducing the frequency of application compared to liquids.
Semi-solid dosage forms are favored in veterinary practices for treating skin ailments, wounds, and localized infections. Their ease of application and sustained release characteristics make them highly effective for pet medicine, ensuring comfort and therapeutic efficiency.
4. Innovative New Veterinary Dosage Forms
The veterinary pharmaceutical industry is always looking to innovate. Recent advancements have led to the development of novel formulations that cater to modern animal care needs.
Emerging New Dosage Forms Include:
✅Slow-Release Collars[1]:
These are designed by incorporating insecticides or repellents into a solid, heat-plastic resin matrix. They:
- Provide prolonged release of the active ingredient.
- Are particularly effective for flea and tick control in pets.
- Enhance compliance by reducing the frequency of application.
✅Microcapsules:
Using advanced polymer technology, active ingredients are encapsulated in tiny capsules (ranging from 1 to 5000 micrometers in diameter).
- They offer controlled release and improved bioavailability.
- Their design ensures protection of the active ingredient from environmental factors until it reaches the target site.
- This technology is often integrated with modern Pharma Packaging solutions to maintain product integrity throughout the supply chain.
These innovative formulations are transforming the veterinary medicine landscape. They meet the evolving needs of pet owners and animal healthcare providers by providing more effective and convenient treatment options.
The Role of Advanced Packaging and Processing Technology
For all these dosage forms, precise manufacturing and packaging are essential. Advanced equipment such as the Solid Preparation Granulation Line plays a crucial role in ensuring uniformity, consistent dosage, and product safety. Here’s how modern technology enhances veterinary medicine production:
Enhanced Uniformity:
Modern granulation systems ensure that each batch of granules or powders has a uniform particle size, which is critical for consistent dosing. This is especially important for solid formulations used in both pet medicine and large-scale veterinary applications.Improved Efficiency in Pharmaceutical Plants:
With automation and precise control systems, manufacturers can optimize production lines to meet high demand while ensuring compliance with stringent quality standards. These advancements benefit laboratories, pharmaceutical plants, and other production environments alike.Reliable Pharma Packaging:
The use of advanced packaging solutions not only protects the product from contamination and environmental degradation but also enhances shelf life. Whether it’s liquid, solid, or semi-solid, proper packaging is key to preserving the efficacy of veterinary formulations.Customization and Scalability:
Modern production lines are adaptable to varying batch sizes, making it possible for both small laboratories and large pharmaceutical plants to manufacture a wide range of veterinary medicine products. This flexibility is critical in today’s dynamic market.
Why It Matters for Veterinary Medicine
Veterinary medicine requires formulations that cater to the specific physiological needs of different animal species. From pet medicine to livestock treatment, the precise control over dosage and release profiles can significantly impact therapeutic outcomes.
🔹Enhanced Animal Health:
High-quality formulations ensure that animals receive the correct dosage, which is vital for effective treatment and recovery. Advanced processing and packaging technologies help maintain these standards, benefiting both pets and farm animals.
🔹Regulatory Compliance:
With rigorous guidelines in place, particularly for products administered to food-producing animals, adherence to quality standards is paramount. Advanced Pharma Packaging and processing equipment ensure that each product meets FDA and GMP requirements.
🔹Market Competitiveness:
In today’s competitive landscape, veterinary pharmaceutical companies must leverage technology to stand out. By utilizing innovative dosage forms and state-of-the-art packaging solutions, manufacturers can achieve better market penetration and customer satisfaction.
Conclusion
In summary, veterinary medicine encompasses a broad range of dosage forms—solid, liquid, semi-solid, and new innovative formulations. Each type has its unique advantages and applications, from ensuring rapid absorption in injectables to providing sustained release in slow-release collars and microcapsules. With advanced equipment like the Solid Preparation Granulation Line and modern Pharma Packaging systems, laboratories and pharmaceutical plants are now better equipped to produce high-quality pet medicine and other veterinary products.
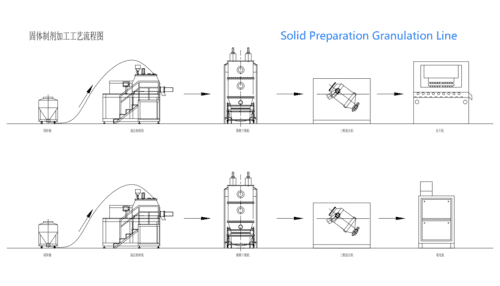
Investing in these technologies not only ensures product consistency and safety but also enhances efficiency across the production chain. Whether you’re a small laboratory or a large-scale pharmaceutical plant, embracing modern manufacturing practices is key to delivering superior veterinary medicine formulations that meet the highest industry standards.
By understanding the four main types of veterinary formulations and the role of advanced packaging technologies, stakeholders in the veterinary pharmaceutical sector can make informed decisions that drive better animal health outcomes and bolster market success.
References
[1] Krüdewagen, E.M., Remer, C., Deuster, K. et al. Chemical Compatibility and Safety of Imidacloprid/Flumethrin Collar (Seresto®) Concomitantly Used with Imidacloprid/Moxidectin (Advocate®, Advantage® Multi) and Emodepside/Praziquantel (Profender®) Spot-on Formulations. Parasitol Res 114 (Suppl 1), 55–80 (2015). https://doi.org/10.1007/s00436-015-4514-z