GrandPack、CPHI & PMEC China 2025で先進的な医薬品包装機械を展示

パイオニア:オリジナル医薬品の知られざる旅
流動床造粒法は医薬品製造にどのような革命をもたらすのでしょうか?
パーティクルダンス
医薬品製造の世界では、精度は単なる目標ではなく、義務です。細かく、しばしば扱いにくい有効成分(API)の粉末から、完璧で均一な錠剤に至るまでの過程は、化学と機械工学の驚異です。何十年もの間、この過程における中心的な課題は造粒、つまり細かい粉末をより強度が高く、密度が高く、予測可能な顆粒に変えるプロセスでした。従来の方法はその目的を果たしてきましたが、現代の医薬品はより高い効率性、制御性、そして品質を求めています。まさに、 流動床造粒 (FBG)が中心的な役割を果たし、プロセスを複数のステップから成る作業からシームレスに統合された芸術形式へと変革します。

この詳細な調査では、この画期的な技術の背後にある原理、優れた固形剤形の製造におけるその大きな利点、そして Grand が提供するソリューションのような最先端の装置が cGMP 製造の新しいベンチマークをどのように設定しているかを探ります。
ダンスの前に: そもそもなぜ顆粒化が必要なのか?
細かい砂で堅固で安定した壁を作ろうと想像してみてください。ほぼ不可能です。粒子は互いにくっつかず、粉塵を発生させ、予想通りに流れません。医薬品の粉末も同じような挙動を示すことがよくあります。原料粉末、特にAPIと添加剤の混合物は、しばしば以下の問題を抱えます。
流動性が悪い: 材料を加工装置を通して錠剤金型に均一に移動させることが困難になります。
高い分離傾向: 混合物内の異なるサイズの粒子が分離し、最終的な錠剤の薬物含有量が不均一になる可能性があり、これは重大な品質不良です。
低い嵩密度: 粉末が「ふわふわ」しすぎていて、耐久性のある錠剤に圧縮するのが困難です。
粉塵発生: オペレーターの暴露や交差汚染のリスクをもたらします。
造粒は、粉末粒子をより大きく均一な凝集体に「接着」することで、これらの問題を解決します。これらの顆粒は液体のように流動し、分離しにくく、圧縮性に優れ、実質的に粉塵が発生しません。
流動層の謎を解き明かす:魔法の仕組み
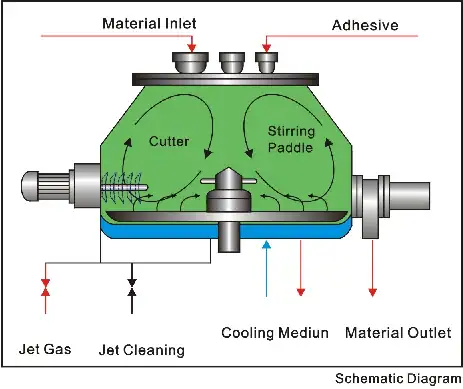
流動層造粒法は、本質的に「シングルポット」または「ワンポット」プロセスです。これは、以下の3つの重要なステップが 混合、造粒、乾燥 これらすべてが同じチャンバー内で行われるため、各段階で別々の機器を必要としていた従来の方法に比べて効率が大幅に向上します。
この洗練されたプロセスを、次のような典型的な高度なシステムを参考にして分解してみましょう。 Grand FLシリーズ沸騰造粒乾燥機.

ステップ1:序章 - 空気の準備と流動化
プロセスは粉末が舞い上がる前から始まっています。強力なファンを通して吸入空気がシステムに取り込まれます。cGMP準拠の機械では、この空気は単なる周囲空気ではなく、綿密に浄化されています。主要機器の仕様書にも記載されているように、 3段階フィルターシステム(プライマリ、ミディアム、高効率HEPA) 標準装備の滅菌システムにより、チャンバーに入る空気は無菌で汚染物質を含まないことが保証されます。浄化された空気は、その後、正確に設定された温度まで加熱されます。
粉末混合物は製品容器に充填されます。加熱・濾過された空気は、容器底部の特殊な空気分配プレートを通して上方に押し上げられます。この空気の速度は厳密に制御されています。空気の速度が上昇するにつれて、粒子に対する空気抵抗が重力に打ち勝つ点に達します。粉末層は膨張し、粒子は空気流中に浮遊し、自由にランダムに動きます。これが 「流動化」状態粉末は沸騰した液体のように振る舞い始め、次のステップに最適な環境を作り出します。これが粒子ダンスです。
ステップ2:主な行為 - バインダーの霧化と凝集
粒子が均質な流体状に懸濁した状態で、液体バインダー溶液を投入します。これは、通常上部に配置された高精度スプレーノズル(トップスプレー造粒)を介して行われます。ノズルはバインダーを微小な液滴に霧化します。
これらの液滴は流動層を通過する際に、懸濁した粉末粒子と接触し、その表面を濡らします。粒子の絶え間ないランダムな運動により、粒子は衝突します。衝突した箇所では、液体バインダーが橋渡し役として働き、粒子同士がくっつきます。 凝集体このプロセスが継続され、粒子は徐々に層ごとに成長し、顆粒を形成します。
ここでのコントロールのレベルは並外れています。 国際薬学ジャーナルバインダースプレー速度、霧化圧力、入口空気温度といった重要なパラメータは、粒度分布や密度といった最終的な顆粒特性に直接影響を及ぼします。最新のPLC制御システムでは、オペレーターがこれらの変数を微調整することで、特定の配合に必要な顆粒特性を正確に実現できます。
ステップ3:フィナーレ - 統合乾燥
所望の顆粒サイズと密度が得られたら、バインダースプレーを停止しますが、加熱流動化空気の流れは継続します。このプロセスは、造粒から乾燥まで、すべて同一チャンバー内でシームレスに移行します。連続した熱風の流れは、顆粒から水分を効率的に除去し、目標の終点(通常は製品温度または乾燥減量(LOD)によって決定されます)に到達します。

ワンステップ乾燥図
乾燥中も顆粒は流動性を維持するため、プロセスは非常に効率的かつ均一です。ホットスポットは発生せず、最終製品の残留水分レベルは非常に低く一定しており、これは製品の安定性と保存期間にとって非常に重要です。
流動床技術の圧倒的な利点
製薬業界における流動床造粒への移行は単なるトレンドではなく、具体的で魅力的なメリットによって推進されています。
比類のない効率性とスループット: シングルポットプロセスのため、処理時間が大幅に短縮されます。ミキサーから別の乾燥機へ湿潤原料を移す必要がないため、時間の節約、人件費の削減、材料ロスの最小化につながります。
優れた顆粒品質と一貫性: 制御された環境により、非常に均一で多孔質の球状顆粒が生成されます。これにより、優れた 流動性 そして 圧縮性これにより、錠剤の重量と硬度がより均一になります。均一な粒度分布(PSD)は、設計による品質(QbD)の基盤となります。
熱に敏感な API に優しい: 流動層乾燥は、従来のトレイ乾燥に比べて高い効率性により、より低温でより短時間の乾燥が可能です。そのため、熱に敏感な有効成分を含む製剤に最適です。
強化された安全性とcGMP準拠: FBGは密閉システムであるため、粉塵の発生を大幅に削減し、作業者の曝露を最小限に抑え、バッチ間の交差汚染を防ぎます。最新の機械には、次のような安全機能が搭載されています。 機械式自動圧力逃し弁 予期しない圧力上昇に対処し、安全な動作環境を確保します。
グランド:造粒の未来をエンジニアリングする
理論を理解することは重要ですが、適切なツールを持つことは別問題です。ここで、エンジニアリングの卓越性への取り組みが最も重要になります。 グランド 創造することに焦点を当ててきた 流動床造粒機 そして GrandPack沸騰造粒乾燥機 プロセスを完璧に実行するだけでなく、自動化とコンプライアンスの最新の進歩も統合します。

彼らのシステム、例えば FLシリーズ現代の医薬品製造の原則を体現しています。
自動化とデータの整合性: 搭載 PLCシステムと直感的なHMIこれらのマシンは完全な自動化とプロセス監視を提供します。バッチデータの保存、エクスポート、印刷機能は記録の維持に不可欠であり、 21 CFR Part 11準拠.
検証済みのクリーニング: 稼働時間を最大化し、純度を確保するため、オプションで WIP(Wash-In-Place)自動洗浄システム 統合できます。これにより、複数の製品を扱う施設において極めて重要な、繰り返し可能で検証可能な洗浄プロセスが実現します。
精密制御: 気流、温度、スプレー速度の可変設定により、堅牢で再現性のある造粒レシピの開発と実行が可能になり、処方者は自信を持って希望の製品プロファイルを実現できるようになります。
造粒を超えて:プラットフォーム技術
流動床技術の汎用性は造粒だけにとどまりません。基本的な装置は、わずかな改造(例えば、ボトムスプレー用のWursterインサートなど)を加えるだけで、以下の用途に使用できます。
粒子コーティング: 味のマスキング、腸溶放出、または持続放出のための機能性コーティングを適用します。
ペレット化: コアシード材料の上に層を構築します。
シンプルな粉末乾燥: 高効率の独立型乾燥機として。
これにより、高品質の流動床プロセッサは多機能プラットフォームとなり、あらゆる医薬品開発や製造施設にとって賢明な投資となります。

結論:パーティクルダンスのパートナー選び
流動層造粒は単なるプロセスではなく、戦略的優位性をもたらします。より効率的、より安全に、そしてこれまで以上に高度な制御下で、高品質の固形製剤を製造するための道筋を提供します。規制基準が厳格化し、複雑な製剤への需要が高まる中、この技術を習得することはもはや必須となっています。
システムを選ぶ際には、基本的な仕様だけにとらわれず、堅牢な構造、インテリジェントなプロセス制御、そしてcGMP原則への深い理解に基づいた設計を重視してください。Grandのようなメーカーと提携することで、機械だけでなく専門知識とサポートも提供し、お客様の施設を今日の課題だけでなく、医薬品製造の未来にも対応できる体制を整えることができます。
よくある質問:グランドの流動床造粒機について
1. Grand の FBG システムはどのようにして製品の純度を保証し、異なるバッチ間の相互汚染を防ぐのでしょうか? Grandのシステムは、cGMP準拠のために徹底的に設計されています。製品の純度は、多面的なアプローチによって保護されています。密閉されたシングルポット処理環境により曝露を最小限に抑え、3段階HEPA濾過システムにより滅菌されたプロセス空気を提供します。オプションの全自動WIP(Wash-In-Place)システムは、検証済みの洗浄サイクルを実行し、すべての残留物を徹底的に除去することで、製品間のキャリーオーバーを確実に防止します。
2. Grand Fluidized Bed Granulator は、熱に敏感な API を含む製剤の処理に適していますか? はい、その通りです。これがこのシステムの大きな強みの一つです。流動化プロセスは熱伝達に非常に優れているため、静的トレイ乾燥機と比較して、顆粒をはるかに低い温度と短時間で乾燥させることができます。システムのPLCは入口空気の温度を正確に制御し、製品温度が臨界閾値を超えることがないようにすることで、敏感な成分の安定性と有効性を維持します。
3. Grand のグラニュレーターは、21 CFR Part 11 などの最新のデータ整合性要件をどのようにサポートしていますか? Grand社の造粒機は、強力なPLCと直感的なHMIを搭載しており、これらがコンプライアンス遵守システムの基盤となります。制御システムは21 CFR Part 11に準拠するように設計されており、安全なユーザーアクセスレベル、電子署名、そしてすべての重要なプロセスパラメータ(時間、温度、エアフロー、スプレー速度など)を記録する機能を備えています。すべてのバッチデータは安全に保存、印刷、エクスポートすることができ、包括的で改ざん不可能な電子バッチ記録と、規制当局による審査のための明確な監査証跡を作成できます。
Grandでビジネスを発展させましょう