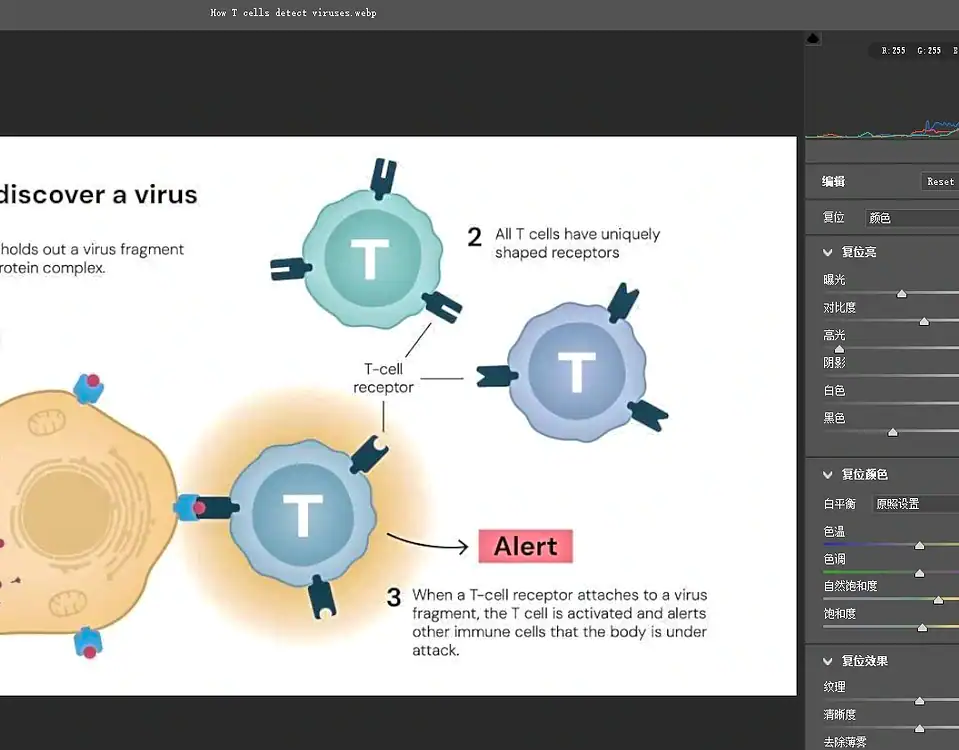
How to Choose an Automatic Capsule Filling Machine?

Boost Efficiency & Cut Costs | Grand Automatic Cartoning Machine
Automatic Tablet Tube Filling Machine Customer Case Study
Introduction: A Detailed URS Is Just the Beginning
A pharmaceutical User Requirement Specification (URS) sets out exactly what a machine must do. Recently, our team at Grandpackmachine received a detailed URS from a pharmaceutical client. The client needed two High-speed automatic tablet tube filling machines with labeling. Key parameters in their URS included: - Speed: 80 tubes per minute.

- Tablet sizes: Four diameters (15 mm, 18 mm, 23 mm, 25 mm).
- Fill counts: Three options (10, 12, 20 tablets per tube).
- Environment: Class D cleanroom conditions.
This URS is very detailed, and meeting it is important. For example, industry guidelines (EU GMP Annex 15) require formal equipment specifications[1]. But for us, a specification sheet is only the start. Our experienced engineers know we must ask deeper questions to deliver a solution that really works.
Key Question 1: Tablet Thickness (Size Is More Than Diameter)
The URS listed four tablet diameters. That is a good start, but what about the tablet thickness? Each pill’s thickness is just as important as its diameter. We asked the client: “What is the thickness of each tablet?”
Why it matters: The machine’s design depends on every tablet dimension. Tracks, feeders, and change parts must match the pill size precisely. Most importantly, thickness determines the stack height. For example, if 20 tablets are stacked in a tube, the total height equals thickness × 20. This stack height affects tube length and the filling mechanism.
Short, clear answers let us confirm how the tablets will stack in the tube. We often see URS documents list diameters but forget thickness. Getting the thickness early means no surprises in mechanical design. Knowing the full 3D size of the tablets ensures the loader, stacker, and tube length are correct.
Key Question 2: Complex Changeovers at 80 bottles/minute
The client’s URS noted 4 tablet sizes × 3 fill counts = 12 possible configurations. We double-checked: “For all 4 tablet sizes, do you need all three filling quantities (10, 12, 20)?” The answer was yes.
This is critical. Running a filling machine at 80 bottles per minute means the tolerances are tiny. High speed makes precise setup essential. In effect, this single machine must handle 12 different runs (4 sizes × 3 fill levels).
Thinking about this, we break it down: - 4 pill diameters (15, 18, 23, 25 mm).
- 3 fill counts (10, 12, 20 tablets).
- Total of 12 unique machine setups.
Each configuration needs its own set of change parts: different chutes, guides, and possibly tubes. We must also consider how long it takes to switch between jobs. A quick changeover is crucial for productivity.
Our action: We will prepare a detailed list of all change parts and designs. We plan to show drawings or videos of how changeovers work. This helps the client see the complexity before buying. They will know exactly how many change sets are needed and how fast they can switch from one tablet size to another.
Key Question 3: Decoding “APCS” for Integrated Quality Control
The URS requested, “Connect to APCS to monitor operating parameters.” We asked the client: “What exactly do you mean by APCS?”

It turned out APCS was not just a simple counter. The client expects on-screen monitoring of tablet weight, potency, contaminants, and seal integrity. In other words, they want an advanced online quality control (QC) system integrated.
This requirement points to high-end features: integrated checkweighers, camera vision inspection, and package leak/seal detectors. Essentially, the customer is asking for a machine that ties into their plant control network and shows quality data in real time.
Next, we asked: “Do you want to use your existing software system, or will we provide the QC software?” The answer will decide our approach:
1. If the client has their plant-wide automation (think Industry 4.0), we may design the filler as a node on their APCS. The machine must communicate data (like weights and defect rates) to their system.
2. If not, we might provide a standalone machine with built-in QC software and displays.
Integrating with an Automated Process Control System (APCS) means true data flow. Integration provides real-time data on every cycle. In fact, integration of an APCS into the plant system allows accurate monitoring and optimization of process parameters[2]. This is a big advantage for production oversight.
In summary, we clarified APCS means the customer wants full QC integration. That is a key technical point. We will confirm exactly how to connect – whether via network protocols or custom APIs – and deliver the right data to the client’s displays.
Key Question 4: Pharma-Grade Documentation for Licensing
The client wrote: “We need suppliers who must provide a full set of documentation on materials so that we can pass the licensing...” In pharma, documentation is everything[3]. We knew this was core to international pharma business.
Every part that touches the product must be compliant (e.g., food-grade plastics, stainless steel 316, etc.). We will supply a full validation document package (DQ, IQ, OQ) to help the client meet regulatory inspections.
This documentation ensures all processes are validated and traceable[3]. Specifically, we will provide:
- Full Material Certificates for all product-contact parts.
- Calibration Certificates for weighing systems and sensors.
- Detailed Drawings and Specifications of the machine.
- Comprehensive Qualification Documents (Design, Installation, Operational Qualification).
These documents form the backbone of the licensing submission. We ensure our machine complies with GMP standards and that every document clearly shows this. In other words, we don’t just sell hardware; we provide paperwork that proves the machine is safe and traceable.
Our solution: Gather all material test reports and specs for the client. Provide certificate copies for every sensor and component. Offer a standard DQ/IQ/OQ pack that covers the whole machine. This way, the client’s validation team can see every requirement is met. By giving these in advance, we remove the risk of a licensing delay.
Key Question 5: Flexibility in International Business
Finally, the URS asked for pricing and payment details: “Give your quotation in RMB... bank details.”
Quality and technical fit are critical, but so is how business is done. A great machine means nothing if the deal can’t close. We see this as part of our service to the customer.
We told the client: as an experienced exporter, we handle complex payments easily. We can quote in the required currency (RMB) and accept payment via VTB Bank or other international channels. The client should not worry about finance details. We make sure paperwork (like proforma invoices) matches their needs.
Being flexible builds trust. The technology may be advanced, but our promise is practical: we support customers through every step. If they need us to work with specific banks or currencies, we adapt. This way, the transaction itself will not be a surprise or obstacle.
Conclusion: We Solve Your URS, Not Just Sell Machines
A URS is more than a shopping list — it’s the start of a conversation. We have shown why we dive into five key areas when decoding a pharma URS for an automatic tablet tube filler. From tablet thickness to machine software, documentation to payment terms, we manage every detail to protect your project’s success.
You don’t have to settle for a supplier who only looks at surface parameters. Send your complex URS to Grandpackmachine. Our engineering team knows the right questions to ask. We turn your requirements into a reliable, validated solution.
Contact us with your URS, and let’s solve it together!
Sources: Industry guidelines and best practices highlight the need for detailed equipment specifications[1], integrated process control for real-time monitoring[2], and thorough validation documentation in pharmaceutical manufacturing[3].