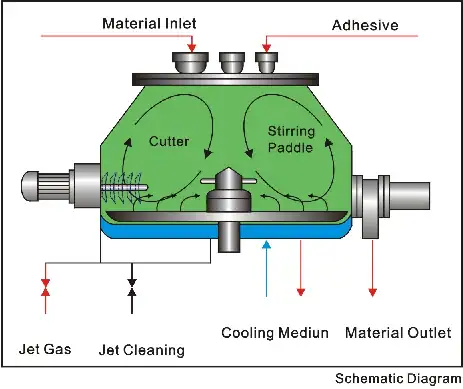
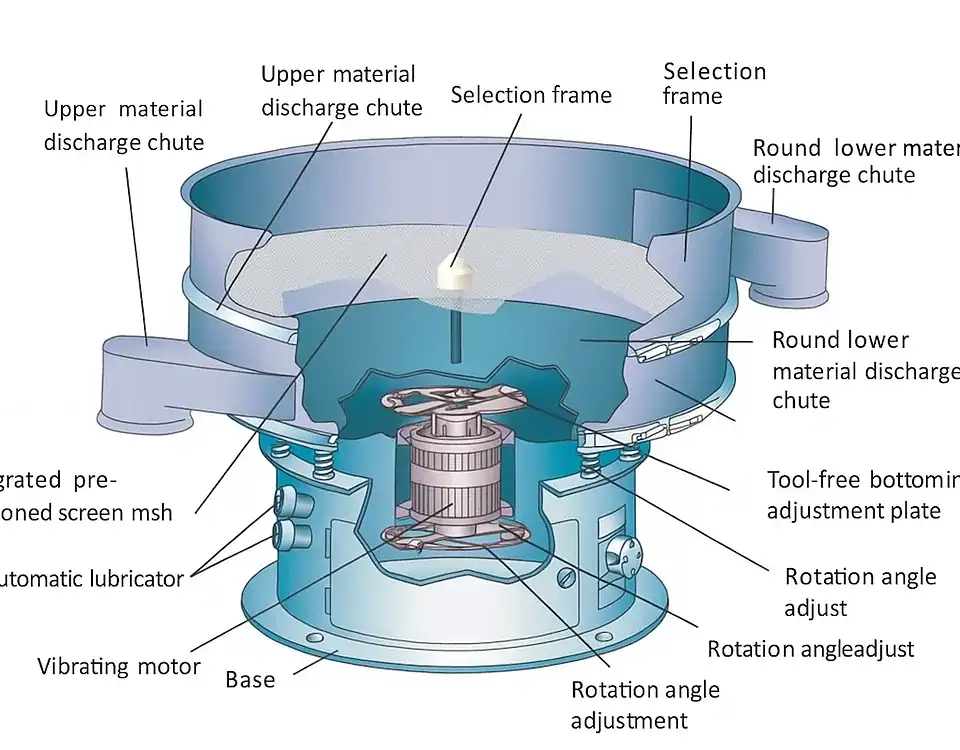
GrandPack Ampoule Filling and Sealing Machine – Efficient Ampoule Filling Line

Need Half a Dose? How to Professionally Divide a Capsule
Pharmaceutical Industry 2025: Drug Launches & Manufacturing Insights
The Pharmaceutical Industry in 2025: A Landscape of Innovation and Transformation
The year 2025 is shaping up to be a pivotal one for the Pharmaceutical Industry. We're witnessing a dynamic interplay of groundbreaking drug launches offering new hope for challenging diseases, alongside the significant market shifts brought about by major patent expirations. Pharmaceutical companies are navigating a landscape ripe with opportunity but also fraught with challenges like mounting regulations and global uncertainties. This evolving environment underscores the critical importance of innovation, strategic planning, and efficient pharmaceutical manufacturing to bring these advancements to patients worldwide.

Navigating a Dynamic Horizon: Opportunities and Hurdles
In 2025, the industry is keenly focused on therapies designed to address critical unmet medical needs. This includes novel treatments for rare diseases, safer non-opioid pain relief alternatives, innovative approaches to tackling obesity and Alzheimer's disease, and new antibiotics to combat the growing threat of antimicrobial resistance. While these advancements are promising, the industry also grapples with escalating costs and patient burdens that impact investor confidence.
Groundbreaking Drug Launches Set to Reshape Treatment Paradigms
Several upcoming therapies are expected to make a significant impact, heralding a new era for medicine by addressing major health concerns.
Targeting Unmet Needs: From UTIs to Alzheimer's
- Gepotidacin (Blujepa): Developed by GSK, this first-in-class oral antibiotic for uncomplicated urinary tract infections (uUTIs) was approved in March 2025. With a novel mechanism that inhibits two essential topoisomerase enzymes, it offers hope against rising antimicrobial resistance. Annual revenue for Gepotidacin Mesylate in the U.S. is projected to reach $197 million by 2033.
- Donanemab (Kisunla): Eli Lilly's monoclonal antibody for Alzheimer's disease targets N3pG beta-amyloid and has shown promise in slowing cognitive decline in early symptomatic patients. Analysts forecast significant market penetration, with Donanemab expected to generate $2.2 billion in annual sales by 2028, and potentially $6.5 billion by 2030 along with its self-injected version.
Addressing Global Health Challenges: Obesity and Acute Pain
- Orforglipron (LY-3502970/OWL833): An oral medication from Chugai Pharmaceutical, licensed by Eli Lilly, for obesity and type 2 diabetes. As an oral pill, it offers potential cost and accessibility advantages over injectables. The global weight-loss drug market, driven by GLP-1 drugs, is expected to soar from $15 billion in 2025 to $94 billion by 2030. Results from a large Phase 3 trial are anticipated to significantly impact the obesity market later this year.
- Suzetrigine (JOURNAVX): From Vertex Pharmaceuticals, this non-opioid oral pain signal inhibitor for moderate-to-severe acute pain received FDA approval on January 30, 2025. Marking the first new class of pain medication approved in over two decades, it offers an effective, well-tolerated, non-addictive option. Sales are projected to reach $2.9 billion by 2030.
Innovations in Rare Diseases and Specialized Care
- Revakinagene taroretcel (Encelto): A gene therapy by Neurotech USA for Macular Telangiectasia type 2 (MacTel 2), approved March 5, 2025, and expected to launch in June 2025. It offers a breakthrough for a condition with no currently approved treatments for its early stages.
- Deutivacaftor/tezacaftor/vanzacaftor (ALYFTREK): Vertex Pharmaceuticals' new triple-combination therapy for cystic fibrosis (CF) in patients aged 6 years and older has received approvals in the UK, USA, and EU. It aims to offer benefits over existing treatments like Trikafta, which generated $8.9 billion in sales in 2023.
The Shifting Tides: Patent Expirations and Market Impact
Beyond new launches, 2025 will also see significant market reshaping due to key patent expirations. This facilitates the growth of generics, altering revenue streams for both branded and generic manufacturers and making treatments more affordable.
High-Stakes Expiries: Entresto, Xarelto, and Jardiance
- Sacubitril/valsartan (Entresto): Novartis's top-selling drug for heart failure, with a patent expiry in July 2025, faces imminent generic competition. Entresto generated $7.822 billion in 2024. The availability of generics is expected to sharply impact Novartis's revenue for this blockbuster.
- Rivaroxaban (Xarelto): Bayer and Johnson & Johnson's anticoagulant saw its first patent expire in 2024, with a secondary patent potentially protecting market exclusivity until 2026, pending court rulings. Xarelto generated approximately €4 billion in sales for Bayer in 2023. Generic versions have already launched, with estimated annual sales for one generic formulation in the U.S. at $446 million.
- Empagliflozin (Jardiance): Boehringer Ingelheim and Eli Lilly's SGLT2 inhibitor for type 2 diabetes, heart failure, and kidney disease faces patent expiry in March 2025. The drug generated $8 billion in global sales in 2023. Generic versions have already launched in markets like India at significantly lower prices, which will undoubtedly impact global revenue streams.

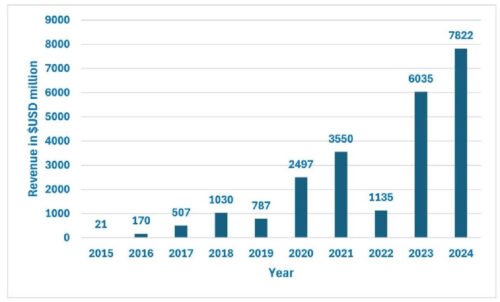
Source: AdisInsight:Entresto market revenue over 2015-2024
The Unseen Engine: Pharmaceutical Manufacturing's Crucial Role
Bringing these innovative therapies to market and ensuring a steady supply of newly genericized drugs relies heavily on robust and advanced pharmaceutical manufacturing. Pharmaceutical companies are continuously investing not only in research and development but also in state-of-the-art manufacturing capabilities to meet global demand and stringent quality standards.
The journey from a promising compound to a patient-ready medication involves sophisticated processes. For instance, the production of oral solid dosages often relies on high-precision Capsule Filling Machine technology and durable table press machines to ensure accurate dosing and tablet integrity. Subsequently, ensuring product stability, safety, and traceability falls to advanced packaging solutions. Automated Blister Machines are vital for packaging tablets and capsules, offering protection and convenience. Specialized firms providing comprehensive packaging lines, sometimes known through industry-specific identifiers like grandpackmachine when referring to integrated and bespoke packaging systems, are crucial in this final, critical step before medications reach the patients who need them.

Looking Ahead: An Evolving Pharmaceutical Ecosystem
The Pharmaceutical Industry in 2025 is characterized by a dual narrative: the excitement of unprecedented innovation bringing new treatments for debilitating conditions, and the economic recalibration driven by the patent cliff for established blockbusters. This environment will continue to push pharmaceutical companies towards greater R&D productivity, strategic lifecycle management, and optimized pharmaceutical manufacturing processes to navigate the path ahead successfully. The ultimate beneficiaries will be patients, who stand to gain access to more effective and, in many cases, more affordable medicines.
Related:Pharmaceutical Industry, 2025 pharma trends, new drug launches, patent expiration, pharmaceutical companies, pharmaceutical manufacturing, grandpackmachine, Blister Machines, capsule Filling Machine, table press, Gepotidacin, Donanemab, Orforglipron, Suzetrigine, Entresto, Xarelto, Jardiance.
References
1.Pharmaceutical Industry Outlook in 2025: New Drug Launches and Patent Cliff.AdisInsight - A comprehensive database of drug information for scientific data from SpringerNature®.