Meilleures recommandations de machines d'emballage sous blister en 2025

Strip Pack ou blister alu-alu : lequel est le plus adapté à votre produit ?
Pourquoi le blister est-il la forteresse la plus vitale de l'industrie pharmaceutique ?

Dans le monde médical, la confiance est primordiale. Un patient place sa confiance dans la certitude que le petit comprimé ou la capsule qu'il s'apprête à prendre est sûr, pur et qu'il produira l'effet thérapeutique escompté. Cette confiance est entretenue par un héros discret et discret de l'industrie pharmaceutique : le plaquette thermoformée. Plus qu'un simple morceau de plastique et de papier d'aluminium, cet omniprésent emballage blister est une merveille de science des matériaux et d'ingénierie de précision : une forteresse miniature conçue pour protéger son précieux contenu d'une multitude de menaces.
Si les bouteilles ont leur place, le passage à emballage sous blister L'adoption de formes posologiques solides a été déterminante, et pour cause. Elle offre un niveau inégalé de protection, d'intégrité et de sécurité des patients. Cet article décortique cette technologie essentielle, explorant la science derrière les matériaux, les avantages qu'elle confère et les avancées technologiques. machines d'emballage sous blister nécessaire pour créer ce gardien essentiel de la médecine moderne.
L'anatomie d'une forteresse : déconstruire le blister
UN plaquette thermoformée Cela paraît simple, mais ses composants sont choisis selon des critères scientifiques rigoureux pour créer un micro-environnement stable pour le médicament.

- Le film de formation (La forteresse de la cavité) : Il s'agit de la cavité en plastique transparent qui abrite le produit. Elle est généralement obtenue par thermoformage, où une feuille de plastique est chauffée et étirée dans un moule. Les matériaux courants sont :
- PVC (chlorure de polyvinyle) : Le produit phare de l'emballage sous blister. Économique, il offre une protection efficace contre l'humidité.
- PVDC (Polychlorure de vinylidène) : Souvent appliqué sur du PVC, le PVDC offre une barrière supérieure à l'humidité et à l'oxygène. Le choix entre le PVC et le PVDC dépend de la sensibilité du médicament. Un critère clé est la Taux de transmission de la vapeur d'eau (WVTR); un WVTR plus faible signifie une meilleure protection contre l'humidité.
- La Porte Impénétrable (La Porte Impénétrable) : Il s'agit du support en aluminium qui scelle le produit dans la cavité. Généralement fabriqué en aluminium renforcé, il offre une barrière quasi parfaite. Taux de transmission de l'oxygène (OTR) et WVTR sont proches de zéro, isolant efficacement le médicament de l'atmosphère. Ceci est essentiel pour prévenir l'oxydation, une voie courante de dégradation des médicaments.
- La forteresse ultime : feuille formée à froid (Alu-Alu) : Pour les médicaments hautement hygroscopiques ou sensibles à la lumière, un thermoformé standard plaquette thermoformée peut ne pas être suffisant. C'est là que alu-alu plaquette thermoformée Ce procédé consiste à former à froid (c'est-à-dire à estamper) un laminé d'aluminium pour obtenir la forme de la cavité. Étant donné qu'il s'agit d'aluminium pur, il constitue une barrière absolue contre l'humidité, l'oxygène et la lumière, offrant ainsi le plus haut niveau de protection possible.
Le « pourquoi » : les quatre piliers de la supériorité de l'emballage blister
L’adoption mondiale de plaquettes alvéolées s’appuie sur quatre avantages indéniables qui répondent directement aux besoins fondamentaux de l’industrie pharmaceutique.
Pilier 1 : Protection et stabilité inflexibles des produits. La fonction première de tout emballage pharmaceutique est de protéger le médicament. La cavité hermétique d'un blister isole chaque dose de l'environnement, la protégeant de l'humidité, de l'oxygène, de la lumière et de la contamination microbienne. Cela préserve l'intégrité chimique et physique du médicament depuis l'usine jusqu'au patient, garantissant ainsi son innocuité et son efficacité jusqu'à sa date de péremption.
Pilier 2 : Garantie de l’intégrité de la dose et de l’observance du patient. Contrairement à un flacon où tous les comprimés sont mélangés, chaque dose dans un plaquette thermoformée se déroule séparément. Cela présente deux avantages majeurs pour la sécurité des patients :
- Précision de la dose : Il empêche les comprimés de se casser ou de s'écailler les uns contre les autres.
- Conformité: Le plaquette alvéolée Les jours de la semaine et autres indications peuvent être imprimés, offrant aux patients un moyen clair et visuel de suivre leur consommation et de respecter le traitement prescrit. Cette fonctionnalité simple s'est avérée considérablement efficace pour améliorer l'observance du traitement.
Pilier 3 : Preuve absolue de falsification. À une époque où les préoccupations en matière de sécurité sont accrues, les preuves d'inviolabilité ne sont pas négociables. plaquette thermoformée Le produit offre cette garantie. Pour accéder au produit, il faut rompre définitivement l'opercule. Il est impossible de l'ouvrir et de le refermer sans laisser de traces évidentes et irréversibles. Cela garantit aux patients et aux professionnels de santé que le médicament n'a pas été compromis.
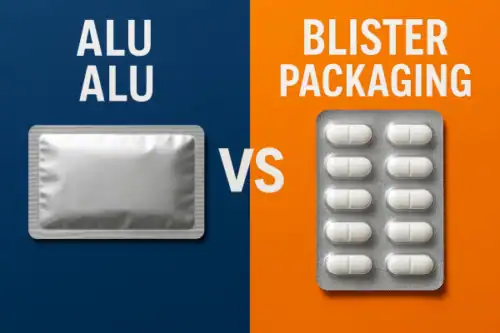
Emballage aluminium ou blister: Choisir la barrière ultime pour votre produit
Pilier 4 : Amélioration de l'information et de la marque Le support en carton d'un plaquette alvéolée, ou la boîte dans laquelle il est placé, offre un espace précieux. Il permet d'imprimer clairement le nom du produit, le dosage, le numéro de lot et la date de péremption. Ces informations sont conservées sur chaque dose, contrairement à un flacon dont l'emballage extérieur peut être jeté.
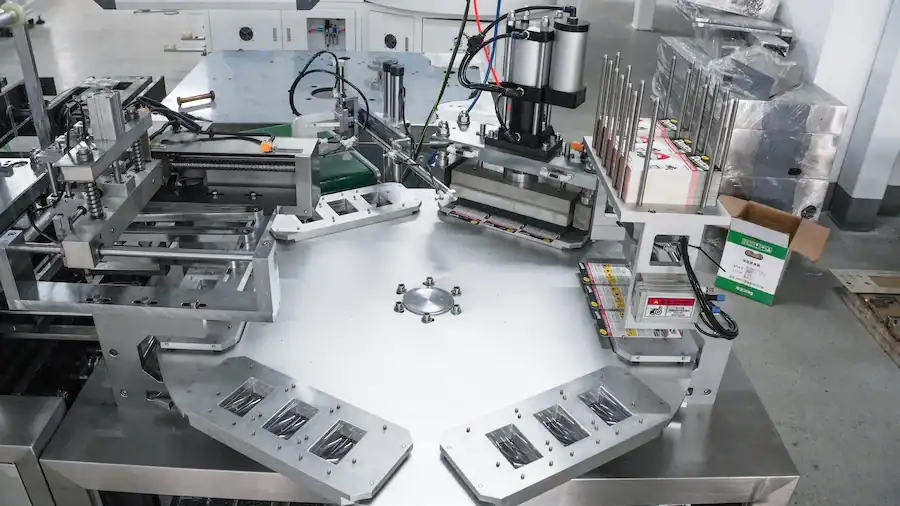
Le « Comment » : un aperçu d'une machine d'emballage sous blister moderne
Créer des millions de ces forteresses parfaites nécessite un système sophistiqué et à grande vitesse. machine d'emballage de comprimés. Le processus, bien que complexe, peut être décomposé en quatre étapes clés exécutées par un seul élément machines d'emballage sous blister.

- Poste de formage : Le film de formage est déroulé et chauffé doucement. Il passe ensuite dans la station de formage où de l'air comprimé le pousse dans un moule refroidi, formant ainsi les cavités précises du moule. emballage sous blister. Pour alu-alu cloques, cette station utilise un tampon pour presser la feuille en forme sans chaleur.
- Station service : Le réseau de cavités nouvellement formé est acheminé vers la station de remplissage. Là, un dispositif d'alimentation dédié (boîte à brosses pour les comprimés, doseur par gravité pour les gélules) dépose avec précision une dose unique dans chaque cavité. Des systèmes de vision peuvent être utilisés pour garantir le remplissage correct de chaque cavité.
- Poste de scellage : Le film d'operculage est mis en contact avec la bande remplie. Ils traversent le machine de scellage d'emballages sous blister Station où un plateau chauffant, souvent moleté, applique une pression et une chaleur précises. Cela permet de coller la couche d'étanchéité de la feuille au film de formage, créant ainsi une étanchéité parfaite autour de chaque cavité.
- Station d'arrivée : Dans la dernière section du machine à emballer sous blisterLa bande scellée est codée avec les informations de lot et de péremption. Elle passe ensuite par des outils de perforation et de découpe qui la séparent en un produit final individuel. plaquettes alvéolées ou des cartes, prêtes pour la prochaine étape d'emballage.

Le Grand Écosystème : Au-delà du blister
Un parfait plaquette thermoformée C'est une réussite cruciale, mais le chemin vers un produit commercialisable n'est pas terminé. C'est là que l'expertise de Grand en matière de lignes complètes et intégrées offre un avantage stratégique.
Le fini sachet blister ou la carte s'écoule de manière transparente à partir de Grandpack machine d'emballage sous blister ligne dans un Grande machine à cartonnerCe système sophistiqué peut alimenter automatiquement les notices, orienter les plaquettes alvéolées et les insérer dans un carton de produit avant de le sceller, créant ainsi le produit final. boîte de remplissage de capsules sous blister.
Mais l'automatisation ne s'arrête pas là. Ces cartons peuvent ensuite être acheminés vers un Grand Case Packer, qui les regroupe et les emballe dans des caisses d'expédition. Enfin, un Grand palettiseur robotisé Nous pouvons empiler ces caisses sur une palette, créant ainsi une charge stable et traçable, prête à être distribuée. En proposant une solution complète, Grand garantit un processus fluide, efficace et fiable, du comprimé brut à la palette finale.
Conclusion : Un engagement envers la qualité, scellé dans du papier d'aluminium
Le plaquette thermoformée est bien plus qu'un simple contenant. C'est un système hautement sophistiqué qui constitue la dernière ligne de défense pour l'intégrité d'un médicament et la sécurité du patient. Son essor est directement lié à sa capacité supérieure à protéger, identifier et sécuriser les doses individuelles de médicaments.
Choisir les bons matériaux et, tout aussi important, les bons machines d'emballage sous blister est une décision cruciale pour tout fabricant pharmaceutique. Une machine fiable et de haute qualité garantit la protection théorique de emballage blister est parfaitement réalisé, des millions de fois. C'est un engagement qualité scellé à chaque emballage.
FAQ : À propos des lignes de remplissage et d'emballage de capsules Grand
- Comment un remplisseur de capsules Grand garantit-il que les capsules sont manipulées en douceur, les rendant parfaites pour le transfert vers une machine d'emballage sous blister à grande vitesse ? Les géluleuses Grand sont conçues pour une manipulation en douceur. Nous utilisons des outils polis et usinés avec précision, ainsi que des mécanismes de transfert optimisés pour minimiser les contraintes mécaniques sur les gélules. Le processus d'éjection est fluide et contrôlé, garantissant que les gélules remplies et verrouillées sont livrées parfaitement intactes et correctement orientées, prêtes à être alimentées en continu. machine à emballer sous blister.
- Un remplisseur de capsules Grand peut-il être synchronisé avec un Grand machine d'emballage sous blister ligne pour un fonctionnement continu ? Absolument. C'est l'un des atouts majeurs de l'écosystème Grand. Nos géluleuses et nos blistereuses sont conçues pour communiquer électroniquement. Elles peuvent être intégrées à une ligne unique et continue grâce à un système de contrôle unifié. La blistereuse peut « appeler » les gélules de la remplisseuse, garantissant ainsi un tampon toujours optimisé entre les machines. Ce fonctionnement synchronisé élimine les goulots d'étranglement et optimise l'efficacité de l'ensemble de la ligne.
- Nous produisons des produits combinés avec deux capsules différentes. Grand peut-il proposer une solution pour les remplir dans une plaquette multi-produits ? Oui, nous pouvons concevoir des solutions sur mesure pour de telles applications. Cela peut impliquer l'utilisation de deux remplisseuses de capsules distinctes et synchronisées, une pour chaque type de capsule, alimentant un système de transfert sur mesure menant à la capsule. machine d'emballage sous blisterLa machine à blisters serait alors programmée pour placer les différentes capsules dans leurs cavités désignées sur le même plaquette alvéolée, offrant une solution complète et automatisée pour vos besoins en produits combinés.
Références
1.Meilleures recommandations de machines d'emballage sous blister en 2025