Yan sızdırmazlık maddesinin performansını ortaya çıkarmak için hangi filmi seçmeliyim?

Grand Hap Pres Makinesi ve Standart: 2025 Alıcı Rehberi
Kapsül Dolum Makinesi Kullanırken Yaptığınız 11 Hata – Hassas Dolum
giriiş
The kapsül dolum makinası katı dozaj formu üretiminin kalbidir. İnanılmaz hassasiyete sahip bir araçtır ve saatte binlerce kez küçük bir kaba tam dozda aktif bileşen vermekle görevlidir. Kusursuz bir şekilde çalıştığında, mekanik ve farmasötik uyumun bir senfonisidir. Ancak, ince hatalar meydana geldiğinde, bu senfoni hızla boşa harcanan kaynakların, tutarsız dozajların ve maliyetli kesintilerin bir kakofonisine dönüşebilir.
Hem yeni hem de deneyimli birçok operatör aynı sinir bozucu engellerle karşılaşıyor. kapsül dolum ekipmanı sadece nasıl açılacağını bilmek değil; toz, kapsül ve makine arasındaki nüanslı etkileşimi anlamaktır. Bu kılavuz, temellerin ötesine geçerek üretiminizi sabote edebilecek en yaygın ve sıklıkla gözden kaçan 11 hatayı ve her seferinde kusursuz sonuçlar için bunları nasıl düzelteceğinizi ortaya çıkarır.

Hata #1: Toz Reolojisini (Tozunuzun "Kişiliğini") Göz Ardı Etmek
Tek bir kapsül doldurulmadan önce, yolculuk tozla başlar. Fiziksel özelliklerini veya reolojisini göz ardı etmek en temel hatadır. Bu sadece "toz" değildir; bir kişiliği vardır. Temel özellikler şunlardır:
- Akıcılık: Kum gibi serbestçe akıyor mu yoksa un gibi topaklanıp köprüleniyor mu? Bu, bilimsel olarak "dinlenme açısı" ile ölçülebilir. Zayıf akışkanlık, tutarsız dolumların ve makine sıkışmalarının birincil nedenidir.
- Yığın ve Sıkıştırılmış Yoğunluk: Bir tozun gevşek yoğunluğu ile çökelmiş (sıkıştırılmış) yoğunluğu arasındaki farkın anlaşılması, doğru dolum ağırlığına ulaşmak için dozaj mekanizmasının kalibre edilmesi açısından kritik öneme sahiptir.
- Sıkıştırılabilirlik: Toz, sıkıştırma tarzı bir dolguda basınç altında nasıl davranır? Bazı formülasyonlar düzgün bir şekilde bir külçeye sıkıştırılırken, diğerleri çok sertleşir veya tutarlılık sağlayamaz.
Çözüm: Formülasyon öncesi analize yatırım yapın. Yaklaşmadan önce tozunuzun özelliklerini karakterize edin kapsül dolum makinasıBu veriler, glidantlar (akışkanlığı iyileştirmek için) veya bağlayıcılar gibi yardımcı maddeler eklemenize ve makinenizi başarıya ulaştırmak için hassas bir şekilde ayarlamanıza olanak tanır.
Hata #2: Kapsül ve Formülasyonda Uyuşmazlık
Tüm kapsüller eşit yaratılmamıştır. bitki tozu kapsülleri, sıvı kapsüller, mikro peletler, vb. Tozunuz için yanlış türü seçmek, yüksek performanslı bir motora yanlış yakıt koymak gibidir. Jelatin ve HPMC (Hipromelloz) olmak üzere iki ana türün farklı özellikleri vardır. Geleneksel tercih olan jelatin kapsüller neme karşı hassastır. Higroskopik (su emen) bir formülasyon, jelatin kabuğundan nemi çekerek onu kırılgan ve çatlamaya meyilli hale getirir.
Çözüm: Kapsülü API ve yardımcı maddelerle eşleştirin. Nem duyarlı formülasyonlar için HPMC kapsülleri kullanın. İlacın etkinliğini azaltabilecek kimyasal etkileşimleri önlemek için seçtiğiniz kapsül malzemesinin aktif bileşenlerinizle uyumlu olduğundan her zaman emin olun.
Hata #3: "Tek Beden Herkese Uyar" Kalibrasyon Yanılgısı
Son parti için işe yarayan ayarların yeni parti için de işe yarayacağını varsaymak felakete davetiye çıkarmaktır. Nem, ortam sıcaklığı ve hatta partiden partiye toz özelliklerindeki küçük değişiklikler bile malzemenin nasıl davrandığını önemli ölçüde etkileyebilir. Hassas dolum dünyasında varsayılan bir ayar yoktur. Bu özellikle kapsül boyutları arasında geçiş yaparken geçerlidir. Bir 4 numara kapsül dolgusu birinden çok farklıdır kapsül dolum makinası boyutu 2.
Çözüm: Kalibre etmek için her bir parti. Dozaj basıncını, makine hızını ve dolum ağırlığını ince ayarlamak için küçük bir test partisi çalıştırmak için zaman ayırın. Önceden yapacağınız bu küçük zaman yatırımı, daha sonra büyük baş ağrılarını ve reddedilen partileri önleyecektir.
Hata #4: Kapsül Bütünlüğünün Ön Dolumda İhmal Edilmesi
Boş kapsüller hareketsiz nesneler değildir. Çevrelerine karşı hassastırlar. Kontrolsüz nem oranına sahip bir odada saklanmaları korkunç sonuçlara yol açabilir. Düşük nem oranı onları kuru ve kırılgan hale getirir, bu da yönlendirme ve ayırma sırasında çatlaklara ve yarıklara yol açar. Yüksek nem oranı onları yumuşak ve yapışkan hale getirir, bu da makinenin kanallarına sıkışmalarına neden olur.
Çözüm: Boş kapsül saklama alanınız için sıkı çevresel kontroller uygulayın. Üreticinin önerdiği saklama koşullarına uyun (genellikle 15-25°C ve 35-65% bağıl nem). Boş kapsülleri aktif bileşenlerinizle aynı özenle işleyin.
Hata #5: Dozaj Mekanizmasının Taleplerini Küçümsemek
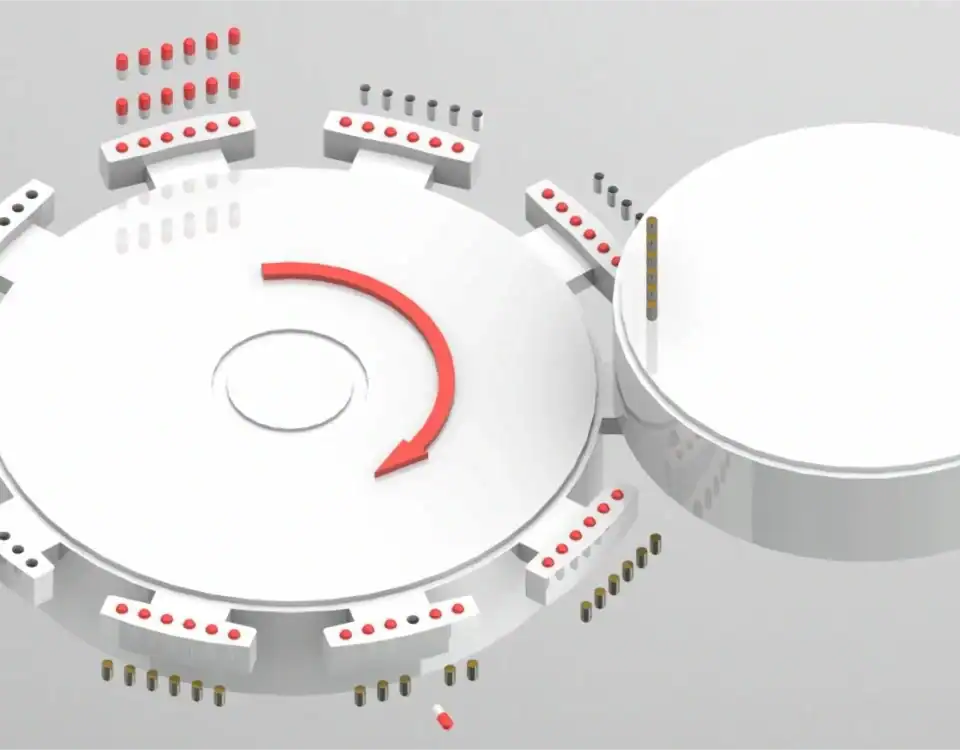
Farklı kapsül dolum makineleri farklı dozajlama teknolojileri kullanın, en yaygın olarak sıkıştırma pimi (doktor) veya helezon sistemleriGrand NJP-Kapsül dolum makinesi serisi). Her birinin kendine özgü güçlü yanları vardır ve uyumlu bir formülasyon gerektirir. Örneğin, bir sıkıştırma pimi sistemi, tozun daha sonra kapsüle aktarılan kohezif bir "kütüğe" sıkıştırılabilme yeteneğine dayanır. Tozunuz çok kabarık veya sıkıştırılamazsa, uygun bir külçe oluşturmayı başaramaz ve bu da kapsüllerin yetersiz doldurulmasına yol açar.

Çözüm: Makinenizin mekanizmasını yakından anlayın. Formülünüzü dozajlama prensibini göz önünde bulundurarak geliştirin. Eğer bir kapsül dolgu 000 Büyük dozlar için, tozun kapatılmadan veya lamine edilmeden büyük ve kararlı bir kütle oluşturabilmesini sağlamak kritik öneme sahiptir.
Hata #6: Eğitimsiz Operatör İkilemi
Son teknoloji kapsül dolum makinası eğitimsiz bir operatörün elinde yüksek hızda hata yaratmak için güçlü bir araçtır. Kurulum, temizlik veya sorun gidermedeki hatalar hasarlı takımlara, uzun süreli duruşlara ve tüm partilerin tehlikeye girmesine yol açabilir.

Çözüm: Kapsamlı, devam eden operatör eğitimine yatırım yapın. Bu bir maliyet değil; büyük bir yatırım getirisi olan bir yatırımdır. Eğitimli operatörler makineyi düzgün bir şekilde kurabilir, sorunları kritik hale gelmeden önce belirleyebilir ve rutin görevleri verimli bir şekilde gerçekleştirerek hem kaliteyi hem de OEE'yi (Genel Ekipman Etkinliği) garanti edebilir.
Hata #7: İşlem İçi Kalite Kontrolünü (IPQC) Atlamak
100.000 kapsüllük bir partinin kalitesini kontrol etmek için sonunu beklemek yüksek riskli bir kumardır. Makine performansındaki küçük sapmalar, sorun fark edilmeden önce binlerce standart dışı kapsüle yol açabilir.
Çözüm: Sağlam bir IPQC protokolü uygulayın. Bu, ağırlık değişimi, uygun kilitleme ve görsel kusurlar gibi kritik kalite özelliklerini kontrol etmek için üretim çalışması boyunca düzenli, tanımlanmış aralıklarla numune almak anlamına gelir. Bu, gerçek zamanlı düzeltmelere olanak tanır ve küçük sapmaların büyük arızalara dönüşmesini önler.
Hata #8: Yetersiz Temizlik ve Çapraz Bulaşma Riski
cGMP ortamında, "temiz" kelimesinin çok özel bir anlamı vardır. Bir makineyi basitçe silmek yeterli değildir. Özellikle güçlü API'lerden kaynaklanan toz kalıntıları sayısız çatlakta saklanabilir. Partiler arasında doğrulanmış bir temizleme prosedürü gerçekleştirmemek ciddi bir çapraz bulaşma riski yaratır.
Çözüm: Sizin için titiz bir temizlik protokolü geliştirin ve doğrulayın. kapsül dolum ekipmanıBu, bir sonraki ürün piyasaya sürülmeden önce makinenin gerçekten temiz olduğunu kanıtlamak için tüm temas parçalarının tamamen sökülmesini, özel temizlik maddelerini ve doğrulama yöntemlerini (sürüntü testi gibi) içermelidir.
Hata #9: Rutin Önleyici Bakımı Göz Ardı Etmek
"Bozulmadıysa tamir etme" ilaç üretiminde tehlikeli bir felsefedir. Planlı bakımı göz ardı etmek aşınmış contalara, yanlış hizalanmış takımlara ve arızalı yataklara yol açar. Bu sorunlar kaçınılmaz olarak maliyetli, planlanmamış duruşlara yol açan felaket niteliğinde arızalara neden olur.
Çözüm: Önleyici bakım programını kutsal olarak kabul edin. Aşınma ve yıpranma parçalarının düzenli olarak yağlanması, incelenmesi ve değiştirilmesi, makinenizin yıllarca güvenilir bir şekilde çalışmasını sağlayarak kullanım ömrünü ve yatırım getirinizi en üst düzeye çıkaracaktır.
Hata #10: Aşırı Dolum/Az Dolum Gerginliği
Amaç her seferinde hedef dolum ağırlığına ulaşmaktır. Aşırı doldurulmuş kapsüller düzgün bir şekilde kapanmayabilir ve kilitlenmeyebilir, bu da sızıntılara yol açabilir. Az doldurulmuş kapsüller gerekli terapötik dozu sağlayamaz. Bu dengeleme eylemi özellikle kapsül boyutlarının uç noktalarında zordur. Küçük bir değişiklik küçük bir kapsül dolum makinası boyutu 5, büyük bir hacim kapsül dolum makinası 000 tutarlı toz paketlemeyi zorlaştırır.
Çözüm: İyi karakterize edilmiş bir tozu (Hata #1) hassas makine kalibrasyonu (Hata #3) ve düzenli işlem içi ağırlık kontrolleri (Hata #7) ile birleştirin. Birçok modern makine, sürekli geri bildirim sağlayan otomatik ağırlık kontrol sistemleri ile donatılabilir.
Hata #11: Aşağı Akış Sürecini Unutmak
Tebrikler, mükemmel kapsüllerden oluşan bir tepsi ürettiniz. İş bitti, değil mi? Yanlış. Yolculuk bitmedi. Mükemmel bir kapsül, sürecin bir sonraki adımında ezilirse veya tehlikeye girerse değersizdir. Kapsül doldurucuyu izole bir ada olarak görmek kritik bir stratejik hatadır.
Çözüm: Tamamlanmış, entegre bir çizgi olarak düşünün. kapsül dolum makinası daha büyük bir iş akışının ilk adımıdır. Grand'da, kapsüllerinizin doldurucudan bir şişeye zahmetsizce aktığı kesintisiz üretim hatları oluşturma konusunda uzmanlaşıyoruz. blister paketleme makinesi, her dozu ayrı ayrı korur. Oradan, otomatik bir karton ve kasa paketleyiciye geçerler. Tüm süreci entegre ederek, manuel taşıma hatalarını ortadan kaldırır, ürün bütünlüğünü korur ve genel verimliliği önemli ölçüde artırırsınız.
Sonuç: Yaygın Hatalardan Sıra Dışı Mükemmelliğe
Bu 11 hatadan kaçınmak, kapsül doldurmayı bir hayal kırıklığı kaynağı olmaktan çıkarıp rekabet avantajına dönüştürür. Tozların bilimine, kapsüllerin bütünlüğüne ve makinenin hassas mühendisliğine saygı duyan bütünsel bir yaklaşım gerektirir. Uygun formülasyonu, titiz süreçleri ve sürekli iyileştirme felsefesini benimseyerek, sadece kapsül üretmenin ötesine geçip mükemmel, tekrarlanabilir ve uyumlu dozaj formları sanatında ustalaşabilirsiniz.
SSS: Grand'ın Kapsül Dolum Makineleri Hakkında
- Grand kapsül dolum makinesi, tutarlı dozajlamayı garantilemek için zor akış özelliklerine sahip tozları nasıl işler? Grand makineler, zorlu tozlar için optimize edilebilen hassas dozajlama sistemleriyle tasarlanmıştır. Sürtünmeyi azaltmak için yüksek kaliteli, cilalı takımlar kullanıyoruz ve sistemlerimiz sıkıştırma basıncı, taret hızı ve toz yatak yüksekliği gibi parametrelerin ince ayarlanmasına olanak tanır. Özellikle zor formülasyonlar için, köprülemeyi önlemek ve dozaj istasyonuna tutarlı bir akış sağlamak için hazne içinde toz karıştırıcılar gibi seçenekler sunuyoruz.
- Grand'ın makineleri, geniş bir kapsül boyutu yelpazesini işleyebilir, 5 numara kapsül dolum makinesi birine kapsül dolum makinası 000? Kesinlikle. Esneklik temel bir tasarım ilkesidir. Makinelerimiz, değiştirilebilir formatlı parçaların kullanımıyla çok çeşitli kapsül boyutlarına uyum sağlayacak şekilde üretilmiştir. Her boyuta özgü takım seti (sıralama blokları, sıkıştırma pimleri ve kapatma istasyonları dahil) en küçük 5 boyutundan en büyük 000 boyutuna kadar her şey için güvenilir elleçleme ve kapatma sağlamak üzere hassas bir şekilde tasarlanmıştır. Değişim, farklı ürün çalışmaları arasındaki kesinti süresini en aza indirerek verimli olacak şekilde tasarlanmıştır.
- Sadece bir dolguya değil, tam bir çizgiye ihtiyacımız var. Grand's nasıl kapsül dolum ekipmanı Blister ambalaj makineleri gibi alt akış makineleriyle entegre edilebilir mi? Grand, komple, anahtar teslimi üretim hatları sağlama konusunda uzmanlaşmıştır. kapsül dolum makineleri diğer ekipmanlarımızla kusursuz entegrasyon için tasarlanmıştır. Kapsüllerin doldurucudan bağlı bir cihaza nazikçe aktarıldığı birleşik bir sistem oluşturuyoruz blister paketleme makinesive sonra bir sonraki adıma kartonlama ve kutulama makinesiTüm hat, merkezi bir kontrol sistemiyle yönetilebilir, böylece senkronize çalışma sağlanır, manuel müdahale en aza indirilir ve servis ve destek için tek bir iletişim noktası sağlanır.