Comment la granulation en lit fluidisé révolutionne la fabrication pharmaceutique ?

Votre guide des 11 meilleurs fabricants de machines d'encartonnage
Pionniers : le voyage inédit des médicaments originaux
Introduction
Dans notre discussion précédente, nous avons exploré le rôle vital des médicaments génériques pour rendre les soins de santé accessibles et stimuler la concurrence sur le marché. Ce sont les piliers du monde pharmaceutique, fournissant des thérapies éprouvées à moindre coût. Mais cela ne raconte qu'une partie de l'histoire. Pour vraiment apprécier l'écosystème de la médecine moderne, nous devons remonter à la source, dans le monde des découvertes scientifiques à enjeux élevés et des risques financiers immenses. Nous devons explorer le cheminement invisible de médicaments d'origine.
Il ne s'agit pas simplement de la « première version » d'un médicament ; ils sont l'aboutissement d'une décennie ou plus de recherches acharnées, d'expériences infructueuses et d'investissements colossaux. Ils représentent le summum de l'innovation pharmaceutique, repoussant les limites du possible dans le traitement des maladies humaines. Cet article explore ce parcours pionnier, d'une simple molécule en laboratoire à un traitement salvateur, et révèle comment l'excellence industrielle est le héros méconnu qui transforme une avancée scientifique en réalité pour les patients du monde entier.
La montagne de la R&D : une ascension de dix ans
Le prix d'un médicament original suscite souvent le débat, mais il reflète un parcours semé d'embûches. Pour chaque médicament commercialisé avec succès, des milliers d'autres candidats sont abandonnés. Le Tufts Center for the Study of Drug Development estime que le coût de développement d'un nouveau médicament sur ordonnance, qui obtient une autorisation de mise sur le marché, s'élève à 142,6 milliards de livres sterling, un chiffre qui explique le taux élevé d'échecs en cours de route.
Ce voyage est un drame en plusieurs actes, témoignage de la rigueur de la méthode scientifique.
Acte I : La phase de découverte
Tout commence par une hypothèse : une nouvelle façon de cibler une maladie. Les scientifiques analysent ensuite des dizaines de milliers de composés chimiques, souvent à l'aide de la robotique de criblage à haut débit (HTS), à la recherche d'un composé prometteur. C'est comme chercher une aiguille dans une botte de foin. Le résultat initial est ensuite optimisé par la chimie médicinale afin d'en accroître l'efficacité et de réduire sa toxicité potentielle, ce qui donne naissance à un composé phare.
Acte II : Le gant préclinique
Avant qu'un médicament puisse être testé sur l'homme, il doit subir des tests précliniques approfondis. Cela implique des tests en laboratoire (in vitro) et animal (in vivo) des études visant à évaluer son profil d'innocuité et son efficacité. Cette phase répond à des questions cruciales : le composé est-il toxique ? Comment est-il absorbé, distribué, métabolisé et excrété (ADME) ? Ces données sont essentielles à la conception d'essais cliniques sûrs sur l'homme et sont compilées dans une demande d'autorisation de mise sur le marché (IND) soumise aux organismes de réglementation comme la FDA.
Acte III : Le marathon des essais cliniques
Si l’IND est approuvé, le médicament entre dans la phase la plus exigeante et la plus coûteuse : les essais cliniques sur l’homme.
Phase I : Un petit groupe de volontaires sains (20 à 100) reçoit le médicament afin d'évaluer sa sécurité, de déterminer une plage posologique sûre et d'identifier les effets secondaires. L'accent est mis uniquement sur la sécurité.
Phase II : Le médicament est administré à un groupe plus large de personnes atteintes de la maladie ciblée (100 à 300) afin de tester son efficacité et d'évaluer plus en détail sa sécurité. Il s'agit du premier véritable test d'efficacité du médicament.
Phase III : C'est l'étape décisive. Le médicament est administré à des milliers de patients (1 000 à plus de 3 000) afin de confirmer son efficacité, de surveiller ses effets secondaires, de le comparer aux traitements courants et de recueillir des informations permettant son utilisation en toute sécurité. Ces essais sont souvent multinationaux et peuvent durer des années.
Seuls environ 121 médicaments TP3T entrant dans les essais cliniques reçoivent l'approbation de la FDA. Les autres échouent en raison d'un manque d'efficacité, de problèmes de sécurité imprévus ou d'une non-viabilité commerciale.
La falaise des brevets et la course contre la montre
Un brevet de 20 ans est la récompense de cet effort herculéen. Cependant, le compte à rebours de ce brevet commence dès le dépôt, souvent dès la phase de découverte. Le processus de R&D absorbant 10 à 15 ans de cette période, le laboratoire de princeps dispose d'une fenêtre d'exclusivité commerciale très étroite – souvent inférieure à dix ans – pour amortir son investissement de plusieurs milliards de dollars avant l'arrivée des génériques sur le marché.
Cette pression immense met en évidence une chose : dès l'approbation, aucune erreur n'est permise. Le processus de fabrication doit être irréprochable, évolutif et prêt pour une production immédiate en grande série.
L'excellence industrielle : le moteur de l'innovation
Une formule moléculaire brillante est inutile si elle ne peut être produite sous une forme galénique stable, efficace et sûre, de manière constante et à grande échelle. C'est là que la synergie entre la science pharmaceutique et l'ingénierie mécanique de pointe devient cruciale. Pour les médicaments princeps, le processus de fabrication n'est pas une réflexion secondaire ; il est développé en parallèle avec le médicament lui-même.
C'est pourquoi les principales entreprises innovantes s'associent à des experts en ingénierie comme Grandiose, dont la technologie est conçue pour répondre aux exigences extrêmes de la mise sur le marché d'un nouveau médicament. La chaîne de production d'un médicament princeps est une extension du laboratoire : un outil garantissant que la précision obtenue en R&D est reproduite dans chaque comprimé ou capsule produit.
Ligne de production intégrée de comprimés de Grand : de la poudre à la perfection
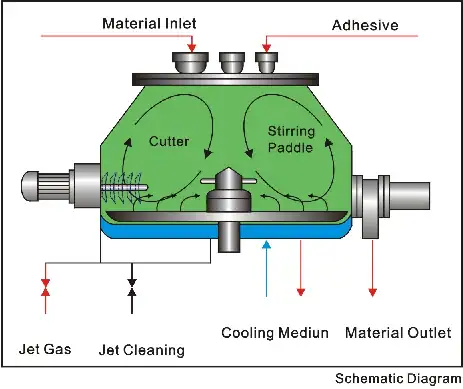
Pour un nouveau comprimé, chaque paramètre compte. L'uniformité du mélange, la précision du dosage, la dureté du comprimé et son profil de dissolution sont autant d'attributs de qualité essentiels définis lors du développement. Ligne de production de comprimés intégrée de Grand est conçu pour contrôler ces variables avec une précision absolue. Un système automatisé gère toutes les étapes, de la granulation et du séchage au mélange, en passant par la compression des comprimés et l'enrobage, avec des points d'intégration de la technologie d'analyse des procédés (PAT) pour un suivi en temps réel. Le système garantit que chaque lot produit pour le lancement commercial est identique à ceux utilisés lors des essais cliniques pivots de phase III, une exigence incontournable pour l'approbation réglementaire.
Ligne complète de production de capsules Grand : une solution clé en main pour des avancées majeures
Pour de nombreuses nouvelles substances chimiques, les gélules constituent une forme galénique idéale. Le processus, de la poudre API à la boîte prête à l'emploi, nécessite un processus parfaitement intégré et hautement automatisé. Grand propose une ligne complète de production de capsules qui gère l'ensemble de ce flux de travail :
Machine de remplissage de capsules : Au début de la ligne, un remplisseur de capsules de haute précision dose avec précision l'API dans chaque capsule, une étape essentielle pour garantir l'uniformité du dosage, en particulier pour les nouveaux médicaments puissants.
Grande machine d'emballage sous blister: Les capsules remplies sont acheminées vers une machine de conditionnement sous blister. Plus qu'un simple conditionnement, il s'agit d'une étape cruciale pour préserver la stabilité du nouveau médicament, le protégeant de l'humidité, de l'oxygène et de la lumière, préservant ainsi sa durée de conservation.
Machine à encartonner (Cartoner) : Les plaquettes thermoformées sont ensuite automatiquement insérées dans les cartons. De nos jours, cette machine est un élément essentiel pour sérialisation, en imprimant des identifiants uniques sur chaque carton pour se conformer aux réglementations de suivi et de traçabilité telles que la loi américaine sur la sécurité de la chaîne d'approvisionnement en médicaments (DSCSA).
Encaisseuse et palettiseur : Enfin, les cartons sont conditionnés en caisses, qui sont ensuite empilées sur des palettes par des systèmes robotisés. Cette automatisation de fin de ligne est essentielle pour gérer le volume important nécessaire au lancement réussi d'un produit.
Pour une entreprise innovante qui court contre la montre en matière de brevets, une ligne entièrement intégrée et validée provenant d'un partenaire unique de confiance comme Grand n'est pas un luxe ; c'est une nécessité stratégique qui accélère le chemin vers le marché.
L'effet d'entraînement : comment les pionniers alimentent toute une industrie
La valeur des médicaments d'origine va bien au-delà du produit lui-même. L'investissement colossal en R&D crée un puissant effet d'entraînement :
Cela fait progresser la science : La recherche approfondit notre compréhension collective de la pathologie des maladies.
Il crée des repères : Le médicament d'origine devient le « médicament de référence » (RLD), la référence absolue en matière de sécurité et d'efficacité à laquelle toutes les futures versions génériques doivent prouver leur équivalence.
Il favorise les nouvelles technologies : Les défis uniques liés à la fabrication d’une nouvelle molécule stimulent souvent le développement de nouvelles techniques de formulation et de technologies de fabrication qui profitent à l’ensemble de l’industrie.
En substance, les médicaments d'origine sont le moteur du progrès. Ils prennent les risques qui ouvrent la voie aux thérapies éprouvées et abordables de demain.
Conclusion : La synergie de la science et de l'acier
Le parcours d'un médicament original est l'histoire de l'ingéniosité humaine, de la rigueur scientifique et d'une persévérance incroyable. C'est un pari risqué, où l'échec est fréquent et où le succès change des vies. Si les génériques jouent un rôle essentiel dans le paysage de la santé, ce sont les pionniers – les développeurs de médicaments originaux – qui tracent la voie vers de nouveaux territoires thérapeutiques.
Cet esprit pionnier est indissociable des outils qui donnent vie aux découvertes. La précision d'une géluleuse, l'opercule protecteur d'un blister et l'intelligence d'une ligne de production intégrée constituent les derniers maillons essentiels de la longue chaîne qui va de la molécule au médicament. Pour les innovateurs prêts à se lancer dans la prochaine grande aventure pharmaceutique, s'associer à des ingénieurs de pointe qui comprennent cette responsabilité profonde est la première étape vers la transformation d'une initiative pionnière en succès mondial.
FAQ : À propos des lignes de production de Grand pour les médicaments d'origine
1. Comment la ligne de production de comprimés intégrée de Grand garantit-elle la cohérence d'un lot à l'autre requise pour une soumission NDA et un lancement commercial ? Les lignes de production de comprimés de Grand sont conçues pour un contrôle et une régularité optimaux. L'ensemble de la ligne fonctionne sous un système de contrôle PLC unifié, garantissant une gestion et une synchronisation précises de tous les paramètres, de la vitesse du granulateur et de la température de séchage à la force de compression de la presse à comprimés et au débit de pulvérisation de l'enrobage. Nous intégrons des points d'accès pour les outils de technologie d'analyse des procédés (PAT), permettant une surveillance et une collecte de données en temps réel. Cet enregistrement fiable des données fournit un enregistrement complet des lots, garantissant la cohérence entre les lots cliniques et commerciaux et répondant aux exigences strictes en matière de données pour une demande d'autorisation de mise sur le marché (NDA).
2. La ligne de production complète de capsules de Grand peut-elle répondre aux exigences de sérialisation et de suivi et de traçabilité essentielles pour les médicaments d'origine ? Oui, absolument. Notre gamme complète de gélules est conçue pour répondre aux normes les plus récentes. Nos encartonneuses et encaisseuses sont conçues pour s'intégrer parfaitement aux principaux systèmes de vision et de sérialisation. Elles assurent la manipulation et le contrôle physiques nécessaires à l'impression, la vérification et l'agrégation d'identifiants uniques (tels que les numéros de série et les codes Data Matrix 2D) sur chaque boîte et caisse, garantissant ainsi une conformité totale aux réglementations telles que la DSCSA aux États-Unis et la FMD en Europe.
3. La validation des procédés et des équipements (QI/QO/QP) est essentielle pour un nouveau médicament à forte valeur ajoutée. Quel soutien Grand propose-t-il dans ce domaine ? Nous comprenons que la validation est une étape cruciale. Grand fournit une documentation complète pour un processus de validation fluide et efficace. Celle-ci comprend des manuels d'équipement détaillés, des schémas électriques et mécaniques, des certificats de matériaux (par exemple, pour les pièces de contact en acier inoxydable 316L) et des protocoles rigoureux de qualification d'installation (QI) et de qualification opérationnelle (QO). De plus, nos ingénieurs terrain expérimentés peuvent intervenir sur site pour accompagner votre équipe dans l'installation, l'exécution des protocoles QI/QO et la formation des opérateurs, simplifiant ainsi considérablement votre parcours vers une ligne de production entièrement qualifiée et prête à la production.
Développez votre entreprise avec Grand