
What are the common packaging of cold medicines?
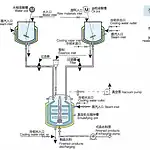
What are cosmetics made of? Homogenizer can do it!
Packaging mechanical structure similar to the 2025 Nobel Prize in Medicine!
Introduction
During China's National Day holiday, the Nobel Prize announcement coincided with the 2025 Nobel Prize in Physiology or Medicine. Two American scientists, Mary E. Brunkow and Fred Ramsdell, and one Japanese scientist, Shimon Sakaguchi, were awarded the Nobel Prize in Physiology or Medicine for their discoveries in peripheral immune tolerance. A careful reading of the award reasons reminded me that Grandpack's pharmaceutical machinery also shares similar principles. Let's briefly discuss this.

On October 6, 2025, at around 17:30 Beijing time, the 2025 Nobel Prize in Physiology or Medicine was awarded to three scientists for their discoveries in peripheral immune tolerance.
What is peripheral immune tolerance?
Peripheral immune tolerance is a key mechanism by which the immune system prevents itself from attacking itself, implemented by regulatory T cells. Together with central tolerance in the thymus, it forms two lines of defense, ensuring that immune cells do not accidentally harm healthy tissue.
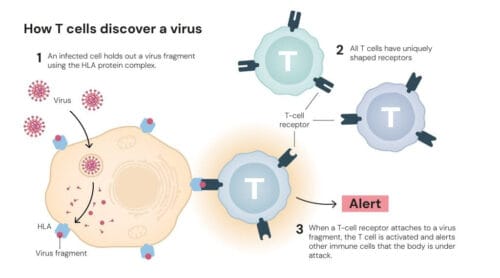
All T cells have special proteins on their surface called T-cell receptors. These receptors can be likened to sensors, allowing T cells to scan other cells to detect whether the body is under attack.
So, what is the connection between the Nobel Prize in Medicine and the safety systems of pharmaceutical machinery?
I understand this is a profound and insightful question. It draws a creative analogy between the core concept of biology—immune tolerance—and the core pursuit of engineering—system safety. We can use a detailed table to compare these two systems:
Immune tolerance system & mechanical safety system
Immune Tolerance System vs. Mechanical Safety System
| Immunology Concept | Corresponding Concept in Pharma/Packaging Machinery | Explanation & Examples |
|---|---|---|
| Self-Tissue (Self) | Machine Body & Normal Operating Parameters | The machine's fixed parts (frame, molds), preset motion paths, rated motor current/torque, normal temperature and pressure ranges. These are what the system must protect. |
| Pathogen/Foreign Object (Non-self) | Materials to be Processed / Abnormal Conditions | Pharmaceutical products, packaging materials (objects to be "attacked"/processed); but also includes misplaced bottles, foreign objects, wrong-sized parts, an operator's hand, etc. |
| Central Immune Tolerance (Thymus) | Design & Manufacturing Stage | The "self" boundaries are defined in the design blueprints and factory calibrations. This includes mechanical hard stops and core safety parameters written into the firmware, serving as the first line of defense. |
| Peripheral Immune Tolerance | Active Safety & Monitoring System during Operation | This is the second line of defense that dynamically and in real-time prevents "self-harm" while the machine is running, functionally identical to peripheral tolerance. |
| Regulatory T-cells (Tregs) | Safety PLC, Sensor Network & Logic Controllers | This is the core of the system. They are the "regulators" responsible for monitoring and "suppressing" dangerous actions. Examples include torque sensors, vision sensors, safety gate switches, and emergency stop buttons. |
| FOXP3 Gene | Core Control Program & Safety Standards (e.g., ISO 13849) | This is the "genetic code" that defines how the "Tregs" function. The safety program in the PLC and hard-coded logic dictate when the machine's actions must be "suppressed" or stopped. |
| Autoimmune Disease (e.g., IPEX) | Catastrophic Equipment Failure (Crashes, Overload Burnout) | When the safety system fails (sensor malfunction, software bug), the machine's actuators (e.g., a tablet press punch) will "attack" its own components (e.g., the die), causing expensive physical damage. |
| Organ Transplant Success Rate | Compatibility & Efficiency of Mold/Part Changeover | Changing molds for different specifications (like an organ transplant) requires the system to "tolerate" and adapt to the new parts. Poka-Yoke (error-proofing) and auto-calibration ensure new parts are correctly identified and accepted, not "rejected" (i.e., causing a collision or producing defects). |
| Cancer Immunotherapy | Predictive Maintenance & AI Vision Systems | This is a more advanced form of "regulation." The system doesn't just react to failures but proactively identifies potential "cancerous cells" (early wear, micro-deviations) through learning and data analysis, intervening before a failure occurs. |
How do "regulatory T cells" in machinery work?
This system is ubiquitous in the equipment you're familiar with:
Pressure sensors continuously monitor punch pressure. Suppose a sudden surge in pressure (potentially damaging the punch and die) due to powder filling anomalies or foreign matter occurs.

In that case, the "regulatory system" (PLC) immediately issues an "inhibit signal," shutting down the machine and preventing it from self-attack.
2. On Grand's auto capsule filling machines:
A visual inspection system checks each workstation. If a damaged capsule shell (an abnormal "not mine") is detected, the system identifies it and rejects it from the subsequent workstation, preventing it from entering the subsequent filling and closing processes. This protects the quality of the subsequent workstations and the final product.

3. On cartoning machines:
If sensors detect that a vial is not properly inserted into a carton, the "regulatory system" stops the pusher, preventing it from executing commands in the incorrect position and potentially damaging the vial, carton, or pusher itself.
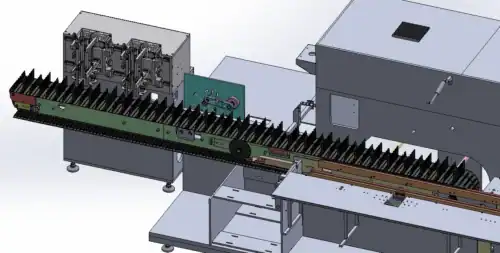
4. On a labeling machine:
If the torque sensor of the servo motor detects an abnormal increase in rotational resistance (perhaps because the label roll is stuck), the system will immediately stop the motor to prevent it from burning out due to overload ("self-damage").

Conclusion
Just as immune tolerance is a sophisticated protective mechanism developed through the long evolution of living organisms, the safety integrity system is an "artificial tolerance mechanism" developed in mechanical engineering after countless failures and accidents.
Its purpose is to enable powerful industrial machines to efficiently complete their tasks while possessing the "intelligence" to avoid accidentally harming themselves, their products, or their operators. With the development of Industry 4.0 and AI technology, this "mechanical immune system" is becoming increasingly intelligent and proactive.
Comparing it to the Nobel Prize may be a bit far-fetched, but the essence of human industry is inextricably linked to scientific and technological progress. The continuous discoveries in biology and medicine always amaze people with the wonders of nature, life, and cells, and humanity is constantly learning and refining its technology.
Of course, Grandpack is no exception. Our factories in Wenzhou and Guangzhou have been deeply engaged in pharmaceutical packaging machinery for many years, continuously refining and optimizing it.
We have developed a variety of practical tablet presses, blister packaging machines, capsule filling machines, and vial and ampoule filling machines.
Our focus on technology and detail has resulted in a wide range of reliable pharmaceutical and packaging machinery, providing pharmaceutical factories with a vital asset. Welcome to consult our Grand brand pharmaceutical machinery. You can quickly submit an inquiry through the form on the right.




