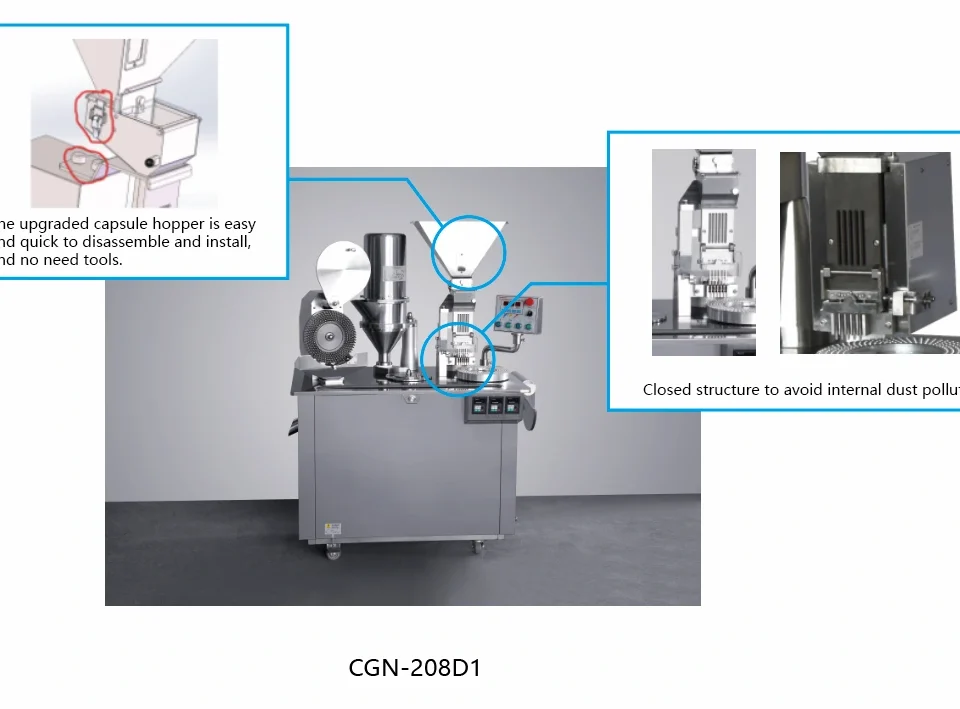
How to Obtain the Perfect Capsule? | Grandpack
The 2026 Flu Surge: How Pharma Machinery is the Frontline Defense Against the H3N2 Variant?
The 2025–2026 influenza season arrived earlier and harder than many expected. Across multiple countries—most notably the United Kingdom, Japan, Australia, and now the United States—hospitals saw sudden surges in admissions. Epidemiologists pinned the spike largely on a rapidly spreading H3N2 variant called subclade K.
That variant carries a cluster of hemagglutinin changes that reduced the immune system’s ability to recognize the virus, producing a crisp reminder: when viruses evolve fast, the pressure shifts from the sequencing lab straight to factory floors that make vaccines, antivirals, and supportive health products.
A man receives a flu shot—the flu has caused numerous infections and hospitalizations in the United States and elsewhere. Source: H.BilbaoEuropa PressGetty
This season pressed manufacturers in two ways. First, public-health teams had to re-characterize circulating viruses and update clinical guidance. Second, production lines faced urgent, large-volume orders for vaccines, oral antivirals, over-the-counter remedies, and immunity-support supplements. The manufacturing response—speed, flexibility, and scale—became as important as the biology itself.
Why subclade K matters?
H3N2 already mutates faster than many other seasonal strains. Subclade K emerged with multiple substitutions in the hemagglutinin spike—mutations located near sites antibodies typically bind. That matters because even one substitution near the receptor-binding site can blunt antibody recognition; K carries several, which together made this variant antigenically distinct from the strains selected for the 2025 vaccine. In short: immunity from prior infection or from the season’s vaccine did not neutralize K as well as we would like.
Public-health reporting from the WHO, national agencies, and academic surveillance teams showed K spreading fast after first appearing in the Southern Hemisphere mid-2025. Its early dominance explains why many countries saw the season start weeks earlier and, in places, last longer than usual.
What that means for vaccines and immunotherapy production?
Traditional egg-based flu vaccine production is reliable but slow. By the time eggs are inoculated, viruses can drift further—an Achilles’ heel when a fast-drifting subclade appears after vaccine strain selection.
Modern platforms give manufacturers more agility.
• Cell-culture and single-use bioreactors scale faster and let producers switch strains with less cross-contamination risk.
• mRNA platforms shorten the path from sequence to product because you can translate a genetic sequence into an mRNA component without growing live virus. Automated LNP (lipid nanoparticle) encapsulation equipment that handles sterile, high-throughput batches becomes a bottleneck-buster in those cases.
• Aseptic filling isolators and high-speed robotic filling lines keep up with demand after bulk drug substance is ready—filling, capping, and labeling hundreds of vials per minute when required.
If sequencing identifies a new dominant variant, factories that use flexible, modular production equipment can shorten the time between sequence and scalable vaccine delivery—often the difference between a controlled uptick and a public-health emergency.
Antivirals and symptom-management drugs: capsule filling at scale
Because vaccine protection was imperfect against subclade K, many people still became ill and sought antiviral therapy and symptomatic relief. That created enormous demand for oral solids—tablets and capsules—which are often the quickest, easiest form to distribute and to scale up.

Modern fully automatic capsule filling lines achieve:
• High throughput—commercial machines commonly reach hundreds of thousands of capsules per hour in continuous runs.
• Tight dosing control—tamping-pin or dosator systems reliably deliver milligram-level accuracy that’s critical for potent antivirals.
• Containment—highly potent APIs require OEB-rated containment and integrated dust extraction to protect operators and avoid cross-contamination during campaign manufacturing.
For manufacturers, the ability to switch a capsule line from one SKU to another with rapid clean-in-place (CIP) cycles and validated changeover procedures was essential to keep pharmacies stocked during peak demand. (See promotion note below for suppliers who specialize in these capabilities.)
Immunity boosters, supplements, and alternative formats
When populations tighten up against a bad season, demand for vitamins, softgels, gummies, and effervescent tablets shoots up. These product families use equipment that borrows from both pharmaceutical and food-grade confectionery engineering.
• Tablet presses monitor compression profiles in real time to avoid defects.
• Continuous rotary softgel lines demand exact temperature control for gelatin ribbons and liquid fills.
• Gummy production lines, built to GMP standards, often mirror candy lines but add dosing and traceability controls.
Manufacturers—especially contract packers—needed high-uptime machinery and rapid analytics to validate each dose’s active content. That was critical because consumers expect consistent potency from immunity products during a crisis.
Packaging and the final mile: anti-counterfeit and cold chain
In a shortage, counterfeiters exploit supply gaps. The packaging line becomes a security checkpoint.
• Alu-Alu blister formats deliver the strongest moisture and oxygen barrier for tablet/capsule stability.

• Serialization and aggregation, driven by high-speed vision systems, print and verify a unique DataMatrix code on every carton to enable track-and-trace across international supply chains.
• Automated cold-chain packaging integrates temperature loggers, insulated shippers, and printed instructions to ensure vaccines retain potency en route.
These systems keep products verifiable, protect integrity during transport, and preserve public trust when supply chains come under stress.
Specialized and high-potency manufacturing
Severe flu cases sometimes require immunosuppressants or other high-potency drugs in ICU settings. Producing these medicines demands isolators, robotic handling, and rigorous environmental controls. Factories running campaign manufacturing had to balance urgent antiviral production with maintaining supplies of immunosuppressants for vulnerable patients—another operational and scheduling challenge during the K wave.
Industry 4.0: digital integration saved time and uptime
Digitally connected plants made a measurable difference. Sensors on capsule fillers, tablet presses, and filling lines gave engineers early warnings of wear or misalignment.
• Predictive maintenance reduced unplanned downtime.
• Real-time quality data sped release decisions.
• Remote parameter adjustments helped scale processes across multiple sites when travel or staffing strained factories.
The K subclade’s pace of change—several amino-acid substitutions accruing within months—meant that manufacturers who had invested in digital twins, automated quality checks, and connected validation workflows could adapt dosing or fill speeds, and get product out faster and safer.
Industry perspective: Helen, Sales Manager at Grand
“In the pharmaceutical field, our machinery covers capsule, drug, and production line filling and packaging,”  says Helen, Sales Manager at Grand. “We provide excellent service to pharmaceutical and health-supplement companies worldwide.
says Helen, Sales Manager at Grand. “We provide excellent service to pharmaceutical and health-supplement companies worldwide.
During the 2025–26 season, customers needed machines that could shift quickly between vaccine adjuncts, antivirals, and consumer supplements. Our modular capsule-filling lines and full packaging suites helped several customers avoid stockouts while meeting regulatory traceability requirements.”

Grand’s hands-on experience shows manufacturers don’t just buy machines; they buy validated, service-backed systems that keep lines running through peak demand.
About Grandpack (brief product note)
Grandpackmachine offers capsule filling production lines, automatic capsule filling machines, tablet presses, blister machines, and end-of-line serialization systems designed for rapid changeover and GMP compliance. Their capsule lines support high containment, in-process weight checks, and OEE monitoring—features that matter under surge conditions.
References:
- Updates for the 2025-2026 flu season
- Kirsebom, F. C. M. et al. Euro Surveill. 30, 2500854 (2025).
- Liu, J. et al. Preprint at medRxiv https://doi.org/10.64898/2026.01.05.26343449 (2026).
- Why is flu so bad this year? Highly mutated variant offers answers https://www.nature.com/articles/d41586-026-00061-6