Top 11 Tube Filling Machine Manufacturers in the World(2025)

Pill counter machine – Pharmaceutical Best Accuracy
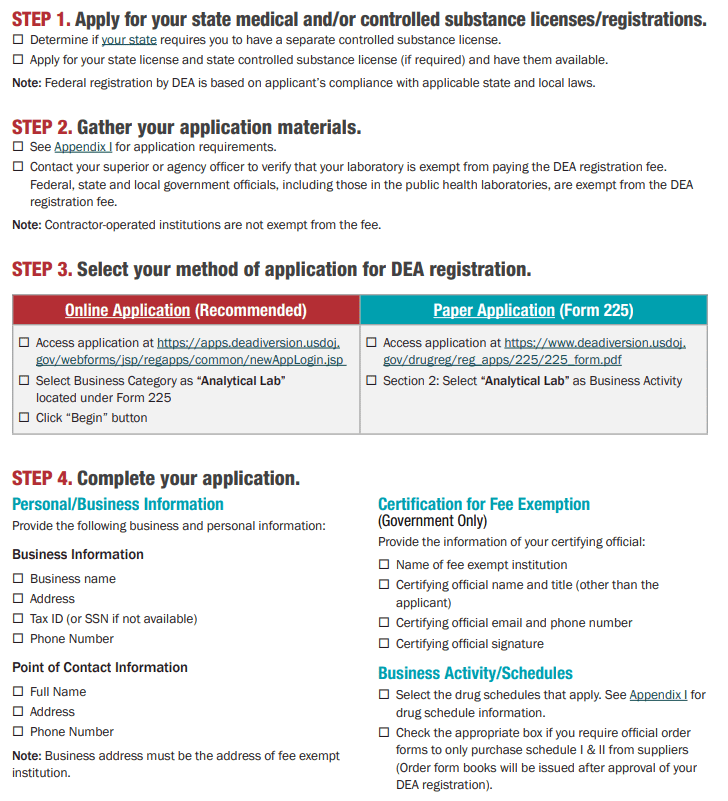
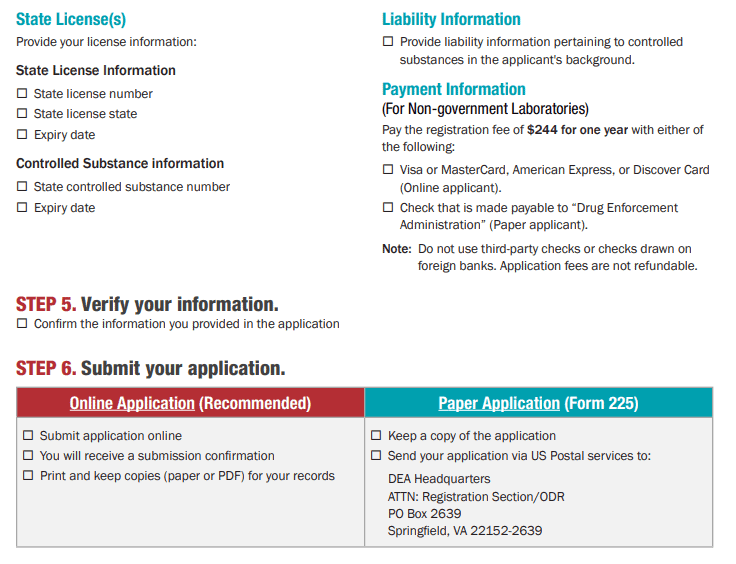
How to Secure Your DEA License for Controlled Machinery Import?
Introduction
Importing equipment such as Capsule filling machines, Tablet presses, and related packaging systems into the United States requires a valid DEA License. Without authorization from the Drug Enforcement Administration (DEA), shipments of controlled machinery can’t clear U.S. Customs. To streamline your application, start at the DEA’s official portal (apps.deadiversion.usdoj.gov/tem/spri spring/login) and register for an account.
Do health supplement companies in the United States need a DEA license to purchase capsule filling machines from Chinese manufacturers?
Answer: Yes, they do!
To be more precise, instead of requiring a long-term DEA registration license like a pharmacy, you must declare to the DEA and obtain a one-time import permit before importing the machine.
The specific explanation is as follows:
Why you need a permit: In the United States, capsule filling machines and tablet presses are classified as "regulated machines" by the DEA, regardless of what you use them to produce. This is because such equipment may be abused to illegally manufacture drugs or counterfeit drugs. Therefore, the DEA strictly controls the import of such machines.
The key lies in the "import" behavior: The focus of supervision is on the "import" link. Even if your final product is a completely legal health product (such as cellulose), as long as you buy it from China and ship it to the United States, you must comply with DEA regulations.
Specific process:
Before the machine is shipped from China, your US company must submit an application form called Form 452 (Machine Import Application) to the DEA.
You need to fill in your company information, Chinese seller information, and detailed specifications of the machine in the form.
The most important step is that you must prove on the form that you are purchasing this machine for "legitimate commercial use", such as producing health supplements.
Only after the DEA reviews and approves your application can the machine be cleared by the US Customs and Border Protection (CBP). Without this pre-approval, the machine is likely to be seized or destroyed by customs after it arrives in the United States.
Regardless of machine size and power, this regulation applies to all types and sizes of capsule filling machines, whether small, low-power, manual, or fully automatic, and must be declared.
In a 2019 study published in the Journal of Pharmaceutical Sciences, researchers observed that organizations with well-structured import compliance programs reduced approval times by up to 30%.¹ By following our clear, active-voice steps, you’ll learn how to complete DEA Form 452 (“Importation of Controlled Machinery”) and track your transaction until you receive your DEA License.


Grand Tablet presses & Capsule filling machines
Step 1: Create Your DEA Online Account & Access Form 452
Visit the DEA Diversion Control Division website and click “Register” if you haven’t already. After you verify your email and set up multi-factor authentication, log in to the Spring system. On the Controlled Machinery homepage, select code 452—the specific form for importing controlled machines. Click “Import” to begin your DEA License application process. Creating an online profile ensures you can revisit your application, save drafts, and download completed forms once the DEA License is granted.
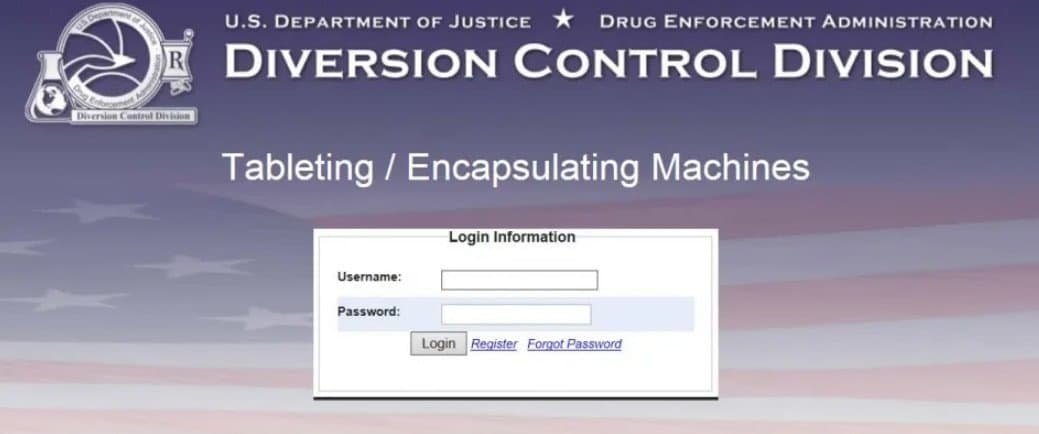
(1). Login page. Enter your username and password and click Login:
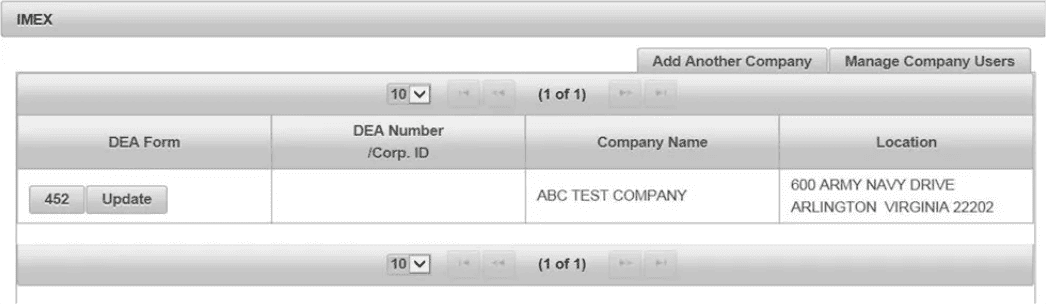
(2). Control machine home page. Click 452
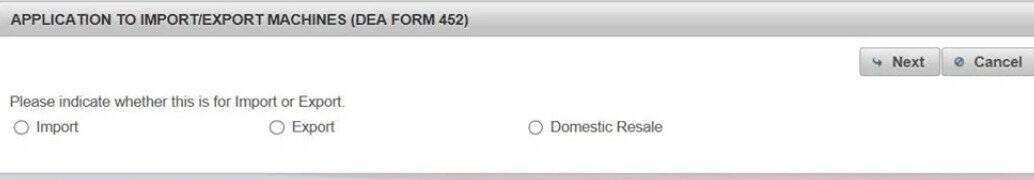
(3). Click "Import" to complete the "Application to Import Machinery" (DEA Form 452). Click "Next"
Step 2: Complete the Importer, Foreign Supplier, and Broker Details
Once you click “Create New DEA Form 452,” the system prompts you to add your company’s information as the importer. Enter your legal business name, address, and contact details exactly as they appear on your corporate documents. Next, click “Add Foreign Shipper” to define the overseas supplier of your capsule filling or sealing machine. Then, under “Add Broker,” fill in your freight forwarder or customs broker’s details. Staying active in these fields prevents common data-entry delays that could stall your DEA License approval.
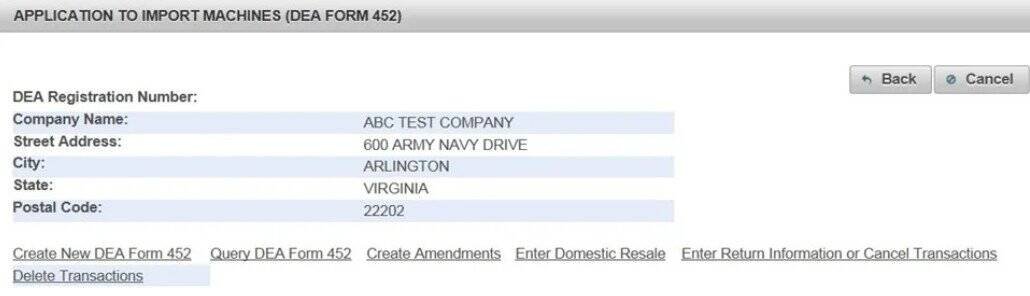
(4). Click Create New DEA Form 452
(5).Click to add a new foreign shipper

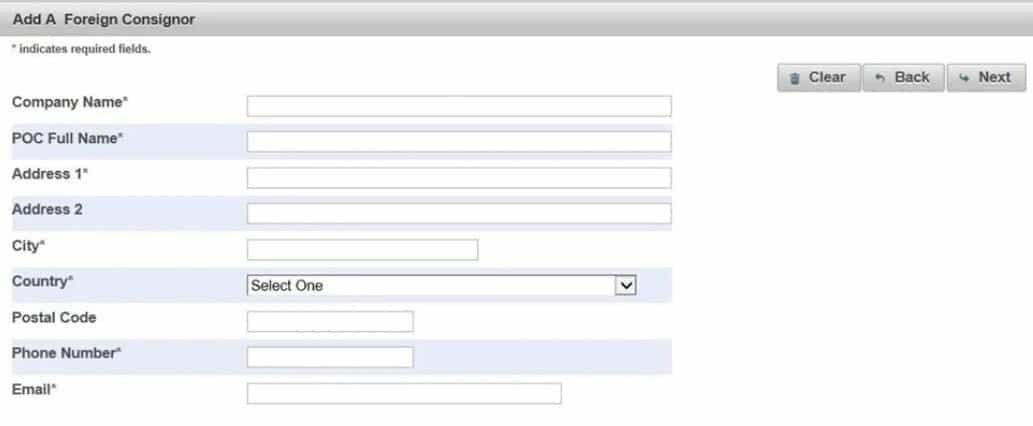
(6).Enter the "Add a Foreign Shipper" fields. Click "Next"
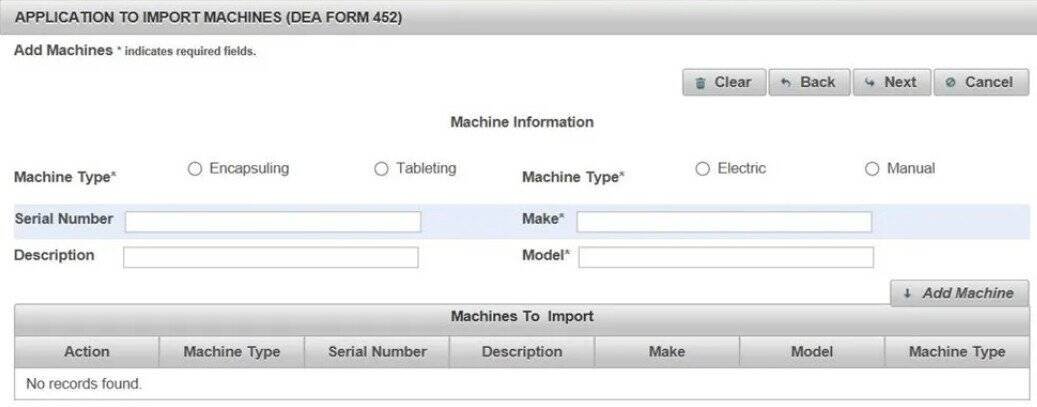
(7).Enter the "Add Machine" fields from the DEA 452 form you want to import. Click "Add Machine". Click "Next"
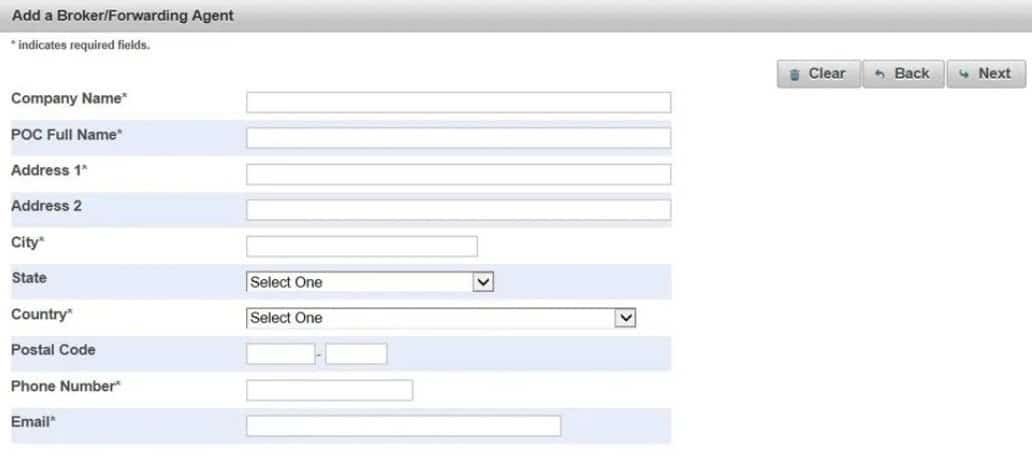
(8).Add New Broker

(9).Fill in the fields to add a Broker/Freight Forwarder
Step 3: Provide Shipment & Machine Specifications
In the “Shipment Information” section, supply the shipment date, port of entry, and Estimated Time of Arrival (ETA). Then click “Next,” and select your import purpose (e.g., “Commercial,” “Scientific,” or “Medical”). For each machine, use the “Add Machine” dialogue to specify model numbers, serial numbers, and description (e.g., “Automatic Capsule Filling Machine with Sealing Module”). Accuracy here is crucial: a 2021 report in the International Journal of Drug Policy highlights that detailed equipment specs reduced review cycles by 25%.² By clearly detailing each machine, you help the DEA review your application faster and issue your DEA License without unnecessary queries.
(10).Fill in the Shipping Information fields
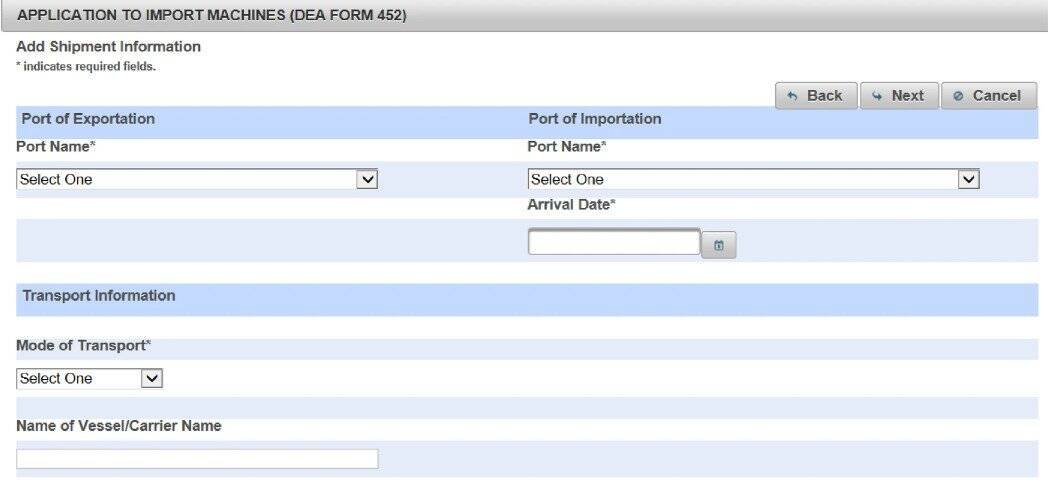
(11).Select the import destination

Step 4: Review, Certify & Submit Your DEA Form 452
Before hitting “Submit,” carefully review every field. Check the certification box that reads: “I certify that the machines listed are necessary for lawful purposes and the information provided is complete and accurate to the best of my knowledge.” Then click “Submit.” You’ll see a network tracking number—write it down. This number lets you log in daily to monitor when your DEA License transaction number appears next to your Form 452 record in the IMEX RCM system.

Submit

Write down the network tracking number

Final Tips & Timeline
Most approvals take 10 days to 4 weeks, depending on case complexity. If your application stalls, call the DEA at (571) 362-3279 or email support through the portal. Keep all correspondence professional and concise. Once your DEA License is issued, you can print the completed Form 452 with its official transaction number and proceed with shipping. Proper planning and attention to detail help ensure your machinery—be it a canister inserter, labeler, or elevator conveyor—clears U.S. entry smoothly.
By following this guide, you’ll navigate the DEA License application process confidently, minimize delays, and focus on expanding your packaging-machine business in the U.S. market.
References
¹ “Efficiency in Controlled Substance Equipment Import Compliance,” J. Pharmaceutical Sciences, 108(2): 450–458 (2019).
² “Impact of Detailed Equipment Descriptions on Regulatory Review Times,” Int’l J. Drug Policy, 92:103–110 (2021).
Web resource reference
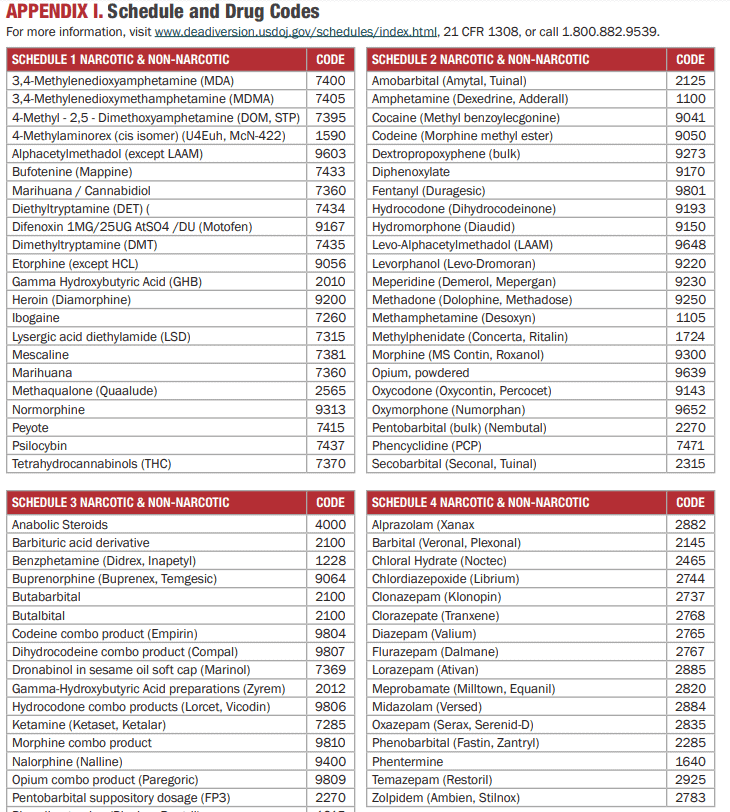
Schedule and Drug Codes